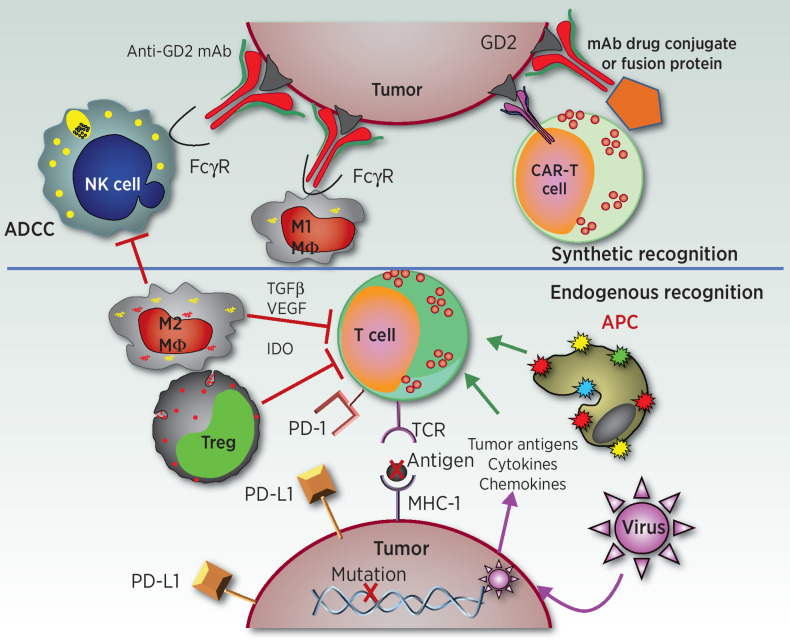
Figure 1.
Endogenous and synthetic recognition involved in immunotherapies for neuroblastoma. This simplified schematic shows some of the relationships between immune cells, molecules, and cancer cells involved in current and developing immunotherapies for neuroblastoma. The synthetic recognition pathways are shown above the horizontal line, and are all shown here as mediated via mAb-induced tumor recognition. The mAb-based tumor-recognition components, shown at the top, include an intact anti-GD2 mAb (at top left) binding to GD2 on the tumor engaging the Fcγ receptor (FcγR) on the NK cell or on the M1 macrophage (MΦ) to activate ADCC. At the top right is that same anti-GD2 mAb, now carrying a “payload.” This payload can be a drug, as an ADC; an immune activator as in a fusion protein, such as an IL2-linked immunocytokine; a radionuclide; or a toxin. To its left is a CAR-T cell that utilizes the ScFv of the anti-GD2 mAb to provide anti-GD2 recognition for the genetically modified T cell. Below the horizontal line are the pathways involved in endogenous recognition, with a central role given to effector T cells. At the bottom of the endogenous T cell is its T-cell antigen receptor (TCR), which on clonally derived T cells can recognize tumor-associated peptides presented by the MHC molecules on the tumor surface. This recognition and T-cell activation can induce effector functions, including cytokine release, activation of innate immune antitumor cells, and direct T cell–mediated tumor cell lysis. Those tumor-associated peptides can be mutation-driven neoantigens (shown here) or germline controlled proteins that have restricted expression to tumor cells, with little or no expression on normal postnatal tissues. To the left of the T cell are endogenous cells that can interfere with T-cell function. One such inhibitory cell is an M2 macrophage (MΦ), which can interfere with antitumor immunotherapy via many pathways, including release of TGFβ, VEGF, and indoleamine 2,3-dioxygenase (IDO). Other myeloid elements, such as myeloid-derived suppressor cells (not shown), can also interfere with effector immune function. Regulatory T cells (Treg) are normally FoxP3+ CD4+ T cells that can directly kill or inhibit the functions of effector T cells. These inhibitory cells can also interfere with NK-cell function (not shown). To the right of the T cell is an antigen-presenting cell (APC), normally a dendritic cell, that picks up and processes tumor antigens and then presents them to T cells to induce an endogenous adaptive immune response. Cytokines and chemokines can help recruit immune cells into the tumor. Certain oncolytic tumor viruses are being injected in some trials to infect the tumor, release more chemokines, and recruit additional immune cells to the tumor microenvironment. The immunosuppressive PD-L1 ligand is one of several checkpoint molecules expressed by tumor cells (and shown here). PD-L1 activates the immune-inhibitory PD-1 receptor on the T cell (shown) and some NK cells (not shown). Not depicted is how anti-PD1 or anti–PD-L1 mAbs (forms of immune checkpoint blockade) can block these inhibitory interactions, enabling T and NK cell functionality in the suppressive tumor microenvironment.