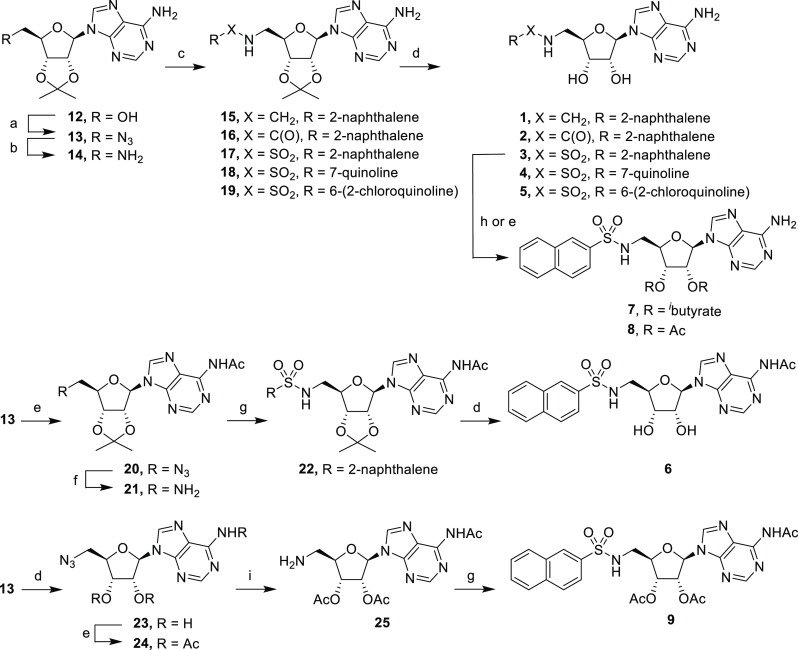
Scheme 1. Synthesis of Adenosine Derivatives.
Reagents and conditions: (a) i. DPPA, DBU, dioxane; ii. NaN3, TBAI, 15-crown-5, dioxane, reflux; (b) H2, Pd/C, MeOH; (c) for 15, 2-naphthaldehyde, NaBH4, MeOH, 5 h, 88%; for 16, 2-naphthoyl chloride, NEt3, CH2Cl2, rt, 18 h, 93%; for 17–19, sulfonyl chloride, NEt3, CH2Cl2, rt, 18 h, 33–93%; (d) TFA/H2O (4:1), rt, 3 h, 38–92%; (e) Ac2O, pyridine, rt, 52–67%; (f) H2, Pd/C, EtOH, 18 h, 69%; (g) 2-naphthalenesulfonyl chloride, NEt3, CH2Cl2, rt, 18 h, 41–44%; (h) isobutyric anhydride, pyridine, rt, 2 h, 29%; (i) H2, Pd/C, dioxane, 18 h.