Scheme 2. Synthesis of C-Nucleosides.
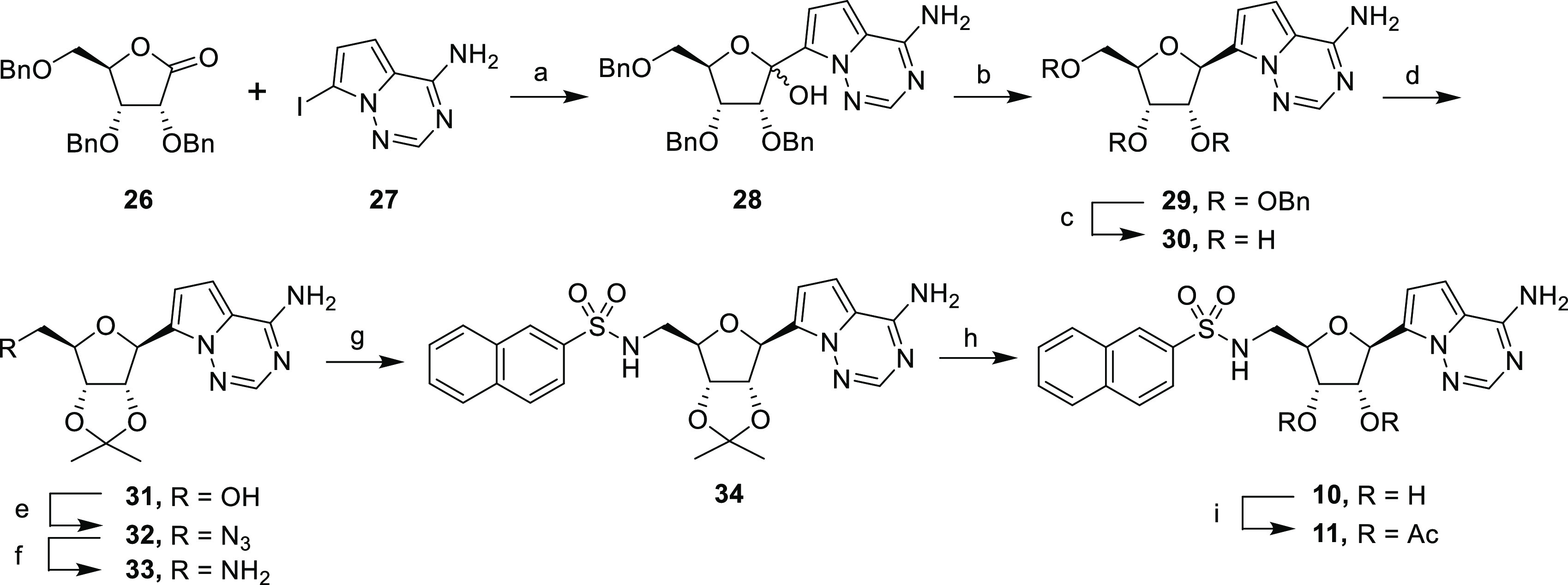
Reagents and conditions: (a) TMSCl, PhMgCl, iPrMgCl·LiCl, THF, 0 °C, 4 h, 42%; (b) BF3·OEt2, Et3SiH, CH2Cl2, 0 °C, 4 h, 87%; (c) BBr3, CH2Cl2, – 78 °C, 2 h, 91%; (d) 2,2-dimethoxypropane, H2SO4, acetone, rt, 87%; (e) i. DPPA, DBU, dioxane, rt, 16 h; ii. NaN3,15-crown-5, 110 °C, 18 h, 82% over two steps; (f) H2, Pd/C, EtOH, 18 h, 95%; (g) 2-naphthalenesulfonyl chloride, NEt3, CH2Cl2, rt, 18 h, 61%; (h) TFA/H2O (4:1), rt, 3 h, 59%; (i) Ac2O, pyridine, rt, 2 h, 35%.
