Box 2.
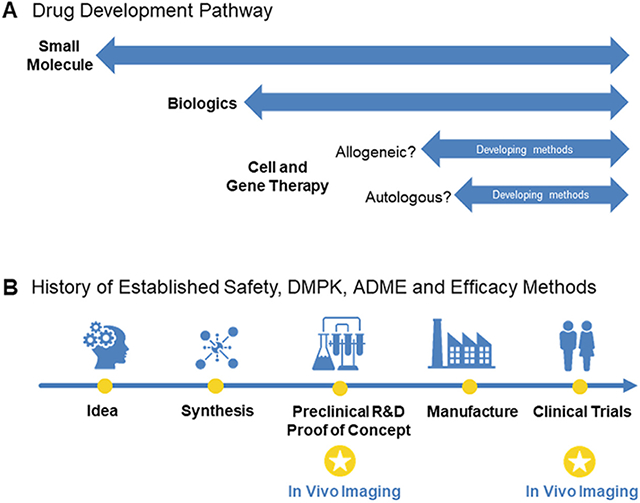
Can imaging add knowledge and experience to the development of cellular therapeutics? (A) In the discovery of small molecules, there are decades of experience and knowledge to draw upon, and these data have shaped the drug discovery pathway, from the idea for a therapeutic target to a licensed product. Many of the in vitro and pre-clinical tests to determine drug safety are also applicable to large molecules and biologics; however, there are fewer established methods for the field of cellular therapies. Arguably, some pre-clinical testing may be relevant, but options are limited for autologous and allogeneic cellular therapies. By and large, the established tools for safety assessment do not transfer well to cellular therapies, but in vivo imaging can play a major part in enabling the gathering of biodistribution data. This will be essential as the field moves to treating solid tumors, where evidence to prove that the cells reach their target will be required, and it is difficult to apply pre-clinical animal data to meet this need for cellular therapeutics. (B) In the development of cellular therapeutics, imaging can help proof-of-concept studies demonstrate mechanism of action. Additionally, imaging stands to play a large role in safety and efficacy in clinical trials. These approaches may help optimize delivery dose and route of administration, demonstrate efficacy of delivery or migration, show how/why some people respond better than others (i.e., responders versus non-responders) and lay the groundwork for co-therapeutics. ADME, absorption, distribution, metabolism and excretion; DMPK, drug metabolism and pharmacokinetics.