Abstract
Objective
Malaria remains endemic in most of sub-Saharan Africa and has a negative impact among pregnant women, resulting in morbidity and poor birth outcomes. The purpose of this study was to assess the relationship between malaria and adverse birth outcomes among prenatal women in the Northern Region of Ghana.
Design
This is a prospective cohort study of singleton pregnancies at 28 weeks of gestational age and above recruited between July 2018 and May 2019 from four public hospitals in the Northern Region of Ghana.
Outcome measures
Low birth weight (LBW), preterm birth and perinatal death.
Results
A total of 1323 pregnant women completed the study out of the 1626 recruited, with an average age of 27.3±5.2 years. The incidence of malaria in this population was 9.5% (95% CI 7.9 to 11.1). After adjusting for newborn admissions to the neonatal intensive care unit, parity, maternal age and glucose-6-phosphate dehydrogenase, women who were exposed to malaria during the third trimester of pregnancy had 2.02 times (95% CI 1.36 to 2.99) higher odds of premature delivery. Furthermore, they had 2.06 times (95% CI 1.09 to 3.93) higher chance of giving birth to babies with LBW, irrespective of their socioeconomic status. With an OR of 1.02 (95% CI 0.26 to 4.01), there was no difference in perinatal mortality between pregnant women with malaria and those without malaria after adjusting for caesarean section.
Conclusion
This study confirms that prenatal malaria increases the odds of both preterm and LBW deliveries. A decisive policy to eradicate or minimise perinatal malaria is needed to contribute to the prevention of LBW and adverse pregnancy outcomes.
Keywords: Antenatal, Maternal medicine, REPRODUCTIVE MEDICINE
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This was a prospective cohort study done to investigate the relationship between maternal malaria in the third trimester and birth outcomes in Tamale, Ghana.
The study provides detailed information on malaria and adverse birth outcomes that is not otherwise readily available due to the challenges of under-reporting and poor record linkage of surveillance data.
Rapid diagnostic tests were used to diagnose malaria during pregnancy as a routine procedure among state-licensed laboratory practitioners, which may be a limitation.
We were unable to account for the effects of malaria on birth outcomes during the early stages of pregnancy.
Introduction
Malaria claimed over 600 000 lives in 2020, with sub-Saharan Africa accounting for 95% of deaths.1 Malaria exposure can be fatal to persons with inadequate immunity, particularly pregnant women and small children. It also has an economic cost to the family and the government, with a direct cost of around $12 billion every year.2
In malaria-endemic areas in sub-Saharan Africa, women face significant risks throughout their pregnancy. Examples of these risks pregnant women are exposed to include low birth weight (LBW), premature birth and spontaneous abortions.3 Prenatal malaria is responsible for 5%–12% of LBW and accounts for between 75 000 and 200 000 infant deaths each year.4 In sub-Saharan Africa, 11 million women were infected with malaria in 2018, resulting in approximately 872 000 newborns born with LBW.5 In 2018, the Central and Western Africa subregions reported the highest prevalence of malaria in pregnant women, each with 35% prevalence. Furthermore, West Africa had the highest frequency of LBW due to malaria.5 In particular, the effect of malaria exposure on fetal growth was observed during the third trimester of pregnancy regardless of period of exposure.6
Malaria cases increased by half a million in Ghana in 2018 compared with the year before.5 Regarding treatment, a research conducted in the War Memorial Hospital in the Upper East Region found that children born to mothers on artemether–lumefantrine intermittent screening and treatment of malaria in pregnancy had a lower risk of malaria than those delivered to mothers on sulfadoxine/pyrimethamine intermittent preventive treatment of malaria in pregnancy (IPTp-SP).7 Yet the prevalence of malaria and poor birth outcomes was 9.0% and 22.2%, respectively, in Kumasi.8 In Navrongo, uptake of intermittent preventive treatment of malaria in pregnancy using sulfadoxine/pyrimethamine at three doses was 76%, while uptake of five doses was 16%, with women who received at least three doses having better health outcomes.8 Given that Ghana is in an endemic malaria zone, these studies highlight implementation gaps and provide information that is useful to improve our malaria prevention policies and programmes. Unfortunately, there is a dearth in the Ghanaian literature relating to the role of malaria in poor birth outcomes in pregnant women in urban settlements in Northern Ghana.
Furthermore, due to insufficient linkages between malaria control and prenatal care data, progress in attaining malaria control among pregnant women has been slow.9 In addition, inconsistencies in data management practices were discovered during a data quality evaluation in several health institutions, posing problems in data reporting, analysis and application.10 Therefore, the precision of aggregate data collected from these facilities through surveillance is compromised by these discrepancies. We designed this prospective cohort research as an independent evaluation of birth outcomes among people with prenatal malaria in light of these difficulties. This study sought to provide considerably more detailed information on the links between prenatal malaria and poor birth outcomes in pregnant women in Northern Ghana.
Methods
Setting
Data for this substudy were drawn from a prospective cohort study that took place in four hospitals in Ghana’s Northern Region. Three of the hospitals are located in Tamale, the Northern Region capital, the fourth largest city in Ghana. The fourth hospital is located in the Savelugu Municipality bordered by Tamale to the west. These areas are located within the Guinea Savannah Belt,11 with little seasonal variations in prevalence, and as such Oheneba-Dornyo and colleagues12 found the prevalence of malaria to be positively correlated with rainfall, with nearly a borderline significance. The Plasmodium falciparum peripheral parasitaemia prevalence in pregnant women in the Northern Savannah Zone ranged between 26% and 13.4% from 2013 to 2019, respectively.13
Design
The study was designed from a parent cohort study that sought to answer the primary research question of whether or not different cooking fuel types influenced pregnancy outcomes and infant respiratory problems.14 15 Therefore, the original sample size calculation was based on the proportion of pregnant women developing the outcome (respiratory symptoms) with cooking fuel type as an exposure. The present study answers a secondary research question about the relationship between prenatal malaria exposure and the risk of adverse birth outcomes. Thus, this study design leverages on the advantages of the large sample size of the original prospective cohort study. As our main results were statistically significant, we assumed that the sample size for this study was reasonable.
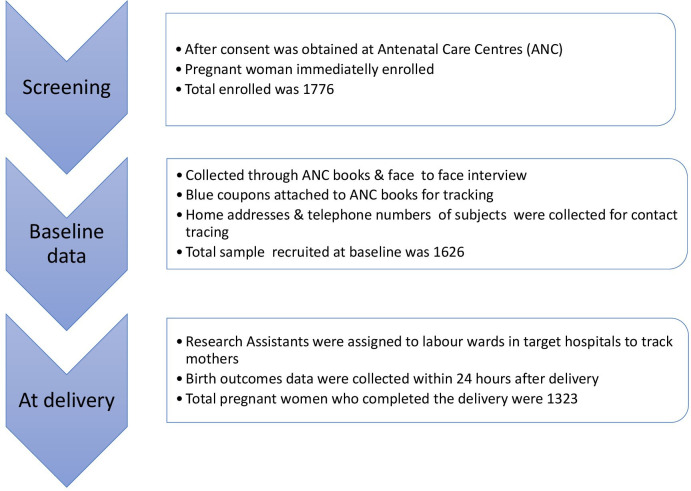
The study recruited pregnant women in their third trimester, who primarily cooked their family meals, were non-smokers and were confirmed to carry singleton pregnancies. The process began in July 2018 and ended in May 2019. The main study was planned with three phases of data collection. At the beginning of the study, women were screened and recruited. In phase 1, during the third trimester, we collected demographics, medical history, exposure data for the primary objective (fuel type) and exposure for secondary objective (malaria). The endpoint for this study was birth outcomes and these data were collected from the labour wards of various hospitals during phase 2. The final part was the phase 3 follow-up that involves collection of newborn data.15
The original study encountered a methodological shortcoming during its implementation as we initially assumed recruited pregnant women will return to the hospitals where they attended antenatal care (ANC) (ie, a recruitment centre) to deliver. However, a few months into the study, we observed that most of them did not return to deliver, and given the project’s limited funding we were unable to follow them up. Therefore, we replaced them with women who strictly agreed to return to the recruitment centre to give birth. This increased our initial sample size from 1472 to 1776, as published in Hussein et al.16 Consequently, we followed up 1323 pregnant women in this study; more details can be found in Hussein et al.15
Procedures
Baseline data were collected at recruitment using a structured questionnaire at the ANC centre of each hospital. Gestational age was routinely ascertained through an ultrasound during ANC; therefore, our study relied on a midwife-validated gestational age. Data on pregnancy outcome were collected at the labour ward of all hospitals by trained research assistants using a predesigned questionnaire. Only 88% of our final sample received at least one sulfadoxine/pyrimethamine (IPTp-SP).
Laboratory procedures
RDT malaria diagnosis
The SD BIOLINE Malaria Ag P.f/Pan rapid diagnostic test kit (RDT) for malaria was used in all hospitals,17 with specificity and sensitivity of 99.5% and 99.7%, respectively. The principal investigator and the research assistants made efforts to observe and monitor adherence to standard testing protocols at each hospital to ensure that they complied with both the manufacturer’s guide and the fundamental laboratory principles for the test. RDT was performed during the third trimester whenever possible to determine whether or not the study participants had parasitaemia in peripheral blood. Still, we were unable to control for possible measurement bias among the laboratory personnel for malaria.
Haemoglobin estimation
All hospitals used a blood analyser to estimate full blood count (FBC), and haemoglobin (Hb) was extracted from the FBC results of all participating pregnant women. Blood sample (5 mL) for Hb estimation was collected into an EDTA tube and mixed with an EDTA anticoagulant. In this study, anaemia was defined as Hb <11.0 g/L.
Glucose-6-phosphate dehydrogenase test
The methaemoglobin reduction test was used in all hospitals to screen pregnant women for glucose-6-phosphate dehydrogenase (G6PD). The test result was reported as no defect/normal, partial defect or full defect.
Data collection
Computer-assisted personal interviewing was used to gather all data, which was done using the KoboCollect Android app. The data collection procedure is described in detail elsewhere.15
Outcomes
The main outcomes of this study were LBW, preterm birth and perinatal death. These were all gathered during delivery in the labour ward of various hospitals. On the seventh day, women were contacted by mobile phone to enquire about the baby’s well-being to ensure that the infant was still alive and to capture neonatal mortality after discharge from the hospital. Preterm birth was defined as <37 weeks gestational age, while LBW was defined as <2.5 kg as previously published.15
Objective
The study aimed to appraise the association of birth outcomes among people with prenatal malaria. The exposure variable was a positive RDT verifying that a pregnant woman had malaria during her third trimester or just before birth.
Statistical analysis
Data were exported from the KoboCollect application database into an MS Excel sheet, and cleaned and transferred to STATA V.13 for analysis. Individual confounders were added into the simple logistic regression model, and a significant variable of >0.05 or near significant was retained in the multiple logistic regressions to evaluate the potential associations between adverse birth outcomes and malaria. For each model, we set out to adjust potential confounders such as maternal age at birth, neonatal admissions at birth, mode of delivery, marital status, parity, G6PD status, genotype, anaemia, socioeconomic status (SES), drinking of alcohol and maternal respiratory condition, and initially added to logistic regression with significance set at p≤0.05. Those with significance were retained in the multiple logistic regression model and the non-significant ones were dropped. Consequently, genotype, anaemia, respiratory condition and drinking of alcohol were all dropped during the initial univariate analysis, which is why some confounders were found in the final model while others were not. Maternal age was, however, non-significant but was retained in the models given its relevance as a confounder and its previously established association with adverse birth outcomes.18 19 For SES, assets from the 2014 Ghana Demographic Health Survey were used to calculate the total assets. We divided the total SES scores into quantiles and considered all scores less than 50 quantiles as poor, from 50 to 75 as moderately rich, and at least 75 or more as rich.15 Missing data of more than 10% from any observation were dropped in order to prevent bias, and a single manual imputation was used to address some missing data based on previous patterns of questions.20 Sensitivity analysis was assessed in both univariate and multiple log binomial regression models compared with logistic regression model used in the main analysis.
Patient and public involvement
There was no patient and public involvement.
Results
Figure 1 shows an elaborate detail of the plan for follow-up during this study, which was published in Husseinet al.15 At baseline, 1626 third trimester pregnant women were recruited, with 1323 women completing the study. The age of pregnant women ranged between 15 and 48 years, and 59.1% were between 25 and 34 years.
Figure 1.
Follow-up plan.
In terms of medical history, 14.8% had a parity of four or more children. The incidence of malaria in this cohort was 9.5% (95% CI 7.9 to 11.1). About 6.4% tested positive for sickle cells, and out of these 50.0% who checked for their genotypes were sickled (SS). About 47.9% of women were anaemic, with Hb levels of less than 11 g/dL within their third trimester of pregnancy, while 4.7% had G6PD full defect (table 1).
Table 1.
Baseline characteristics of pregnant women
| Variables | Delivery period |
| Frequency (%), N=1323 | |
| Maternal age (mean±SD) | 27.3±5.2 |
| 15–24 | 394 (29.8) |
| 25–34 | 782 (59.1) |
| 35–48 | 147 (11.1) |
| Marital status | |
| Married | 1310 (99.0) |
| Unmarried | 13 (1.0) |
| Ethnicity | |
| Mole Dagbani | 1274 (96.3) |
| Others | 49 (3.7) |
| Maternal education | |
| Primary/no education | 997 (75.4) |
| JHS/middle school | 147 (11.1) |
| SHS/technical/vocational | 89 (6.7) |
| At least diploma | 89 (6.8) |
| Maternal occupation | |
| No employment | 302 (22.9) |
| Trader | 834 (63.1) |
| Labourer | 74 (5.6) |
| Factory/industry | 24 (1.8) |
| Formal employment | 87 (6.6) |
| Socioeconomic status | |
| Poor | 473 (35.8) |
| Moderately rich | 439 (33.2) |
| Rich | 411 (31.1) |
| Residence | |
| Urban | 762 (57.6) |
| Rural | 561 (42.4) |
| Housing | |
| Separate house | 68 (5.14) |
| Semidetached | 43 (3.25) |
| Compound house (sandcrete) | 850 (64.25) |
| Compound house (mud) | 362 (27.36) |
| Alcohol | |
| Yes | 4 (0.3) |
| No | 1319 (99.7) |
| Medical history | |
| Parity | |
| First pregnancy | 633 (47.9) |
| 2–3 pregnancies | 494 (37.3) |
| 4 or more pregnancies | 196 (14.8) |
| Malaria | |
| Positive | 125 (9.5) |
| Negative | 1198 (90.5) |
| Sickle cell | |
| Positive | 77 (6.4) |
| Negative | 1120 (93.6) |
| Genotype | |
| Hb AS | 24 (36.4) |
| Hb SC | 9 (13.6) |
| Hb SS | 33 (50.0) |
| Anaemia | |
| <11 g/L | 582 (47.9) |
| ≥11 g/L | 633 (52.1) |
| Sulfadoxine/pyrimethamine usage | |
| Yes | 1137 (88.9) |
| No | 154 (11.9) |
| Glucose-6-phosphate dehydrogenase | |
| No defect | 1029 (86.9) |
| Partial defect | 98 (8.3) |
| Full defect | 56 (4.7) |
| Respiratory condition | |
| Yes | 10 (0.8) |
| No | 1313 (99.2) |
Hb, haemoglobin; JHS, Junior High School; SHS, Senior High School.
The incidence of preterm birth among women with malaria was 52.0%. Moreover, the prevalence of LBW was 10.4% among women with malaria and 5.1% among women without malaria. In both mothers with and without malaria, newborn deaths and live births were equally 1.6% (table 2).
Table 2.
Incidence of pregnancy outcome and malaria
| Pregnancy outcomes, N=1323 | Negative | Positive | Total |
| Frequency (%) | Frequency (%) | ||
| Preterm birth | |||
| Term | 780 (65.1) | 60 (48.0) | 840 |
| Preterm | 418 (34.9) | 65 (52) | 483 |
| Birth weight | |||
| Normal birth weight | 1137 (94.9) | 112 (89.6) | 1249 |
| Low birth weight | 61 (5.1) | 13 (10.4) | 74 |
| Type of delivery | |||
| Live birth | 1176 (98.4) | 123 (98.4) | 1299 |
| Neonatal mortality | 19 (1.6) | 2 (1.6) | 21 |
Pregnant women with malaria had 2.02 times (95% CI 1.39 to 2.93) increased odds of preterm birth compared with those without malaria after adjusting for parity, maternal age, G6PD deficiency and neonatal admission (table 3).
Table 3.
Logistic regression of preterm and malaria
| Preterm | Crude OR (95% CI) | P value | Adjusted OR (95% CI)* | P value |
| Malaria | ||||
| No | 1 | 1 | ||
| Yes | 2.02 (1.39 to 2.93) | <0.001 | 2.02 (1.36 to 2.99) | <0.001 |
| Parity | ||||
| First pregnancy | 1 | 1 | ||
| 2–3 pregnancies | 0.83 (0.65 to 1.07) | 0.147 | 0.79 (0.61 to 1.03) | 0.087 |
| 4 or more pregnancies | 0.59 (0.42 to 0.85) | 0.004 | 0.62 (0.42 to 0.93) | 0.021 |
| Maternal age (years) | ||||
| 15–24 | 1 | |||
| 25–34 | 1.23 (0.96 to 2.85) | 0.102 | 1.35 (1.02 to 1.77) | 0.034 |
| 35–48 | 0.75 (0.49 to 1.13) | 0.170 | 0.92 (0.57 to 1.48) | 0.720 |
| G6PD | ||||
| Normal | 1 | 1 | ||
| Partial defect | 1.55 (1.02 to 2.35) | 0.039 | 1.54 (1.01 to 2.37) | 0.047 |
| Full defect | 1.07 (0.61 to 1.87) | 0.803 | 0.98 (0.54 to 1.76) | 0.934 |
| Neonatal admission at birth | ||||
| No | 1 | 1 | ||
| Yes | 2.10 (1.17 to 3.79) | 0.004 | 1.98 (1.09 to 3.60) | 0.025 |
*Significant confounders adjusted in the multiple log binomial model: parity, maternal age, G6PD and neonatal admissions at birth.
G6PD, glucose-6-phosphate dehydrogenase.
Furthermore, pregnant women with malaria had 2.06 times (95% CI 1.09 to 3.93) increased odds of LBW compared with those without malaria after adjusting for parity, maternal age and SES (table 4).
Table 4.
Logistic regression of LBW and malaria
| LBW | Crude OR (95% CI) | P value | Adjusted OR (95% CI)* | P value |
| Malaria | ||||
| No | 1 | 1 | ||
| Yes | 2.04 (1.16 to 3.61) | 0.014 | 2.06 (1.09 to 3.93) | 0.027 |
| Parity | ||||
| First pregnancy | 1 | 1 | ||
| 2–3 pregnancies | 1.21 (0.71 to 2.06) | 0.480 | 1.32 (0.76 to 2.28) | 0.322 |
| 4 or more pregnancies | 1.72 (0.91 to 3.23) | 0.093 | 2.13 (1.03 to 4.39) | 0.041 |
| Maternal age (years) | ||||
| 15–24 | 1 | |||
| 25–34 | 0.79 (0.47 to 1.32) | 0.373 | 0.70 (0.41 to 1.22) | 0.210 |
| 35–48 | 0.72 (0.30 to 1.71) | 0.457 | 0.45 (0.17 to 1.19) | 0.109 |
| Socioeconomic status | ||||
| Poor | 1 | 1 | ||
| Moderately rich | 0.57 (0.33 to 0.99) | 0.050 | 0.60 (0.34 to 1.07) | 0.082 |
| Rich | 0.52 (0.28 to 0.93) | 0.028 | 0.54 (0.29 to 0.98) | 0.044 |
*Significant confounders adjusted in the multiple log binomial model: parity and socioeconomic status.
LBW, low birth weight.
Lastly, with an OR of 1.02 (95% CI 0.26 to 4.01), there was no difference in perinatal mortality between pregnant women with malaria and those without malaria after adjusting for caesarean section. Women who underwent caesarean section had a five times greater risk of perinatal death than those who did not have caesarean section (table 5).
Table 5.
Logistic regression of perinatal mortality and malaria
| Perinatal mortality | Crude OR (95% CI) | P value | Adjusted OR (95% CI)* | P value (adjusted models) |
| Malaria | ||||
| No | 1 | 1 | ||
| Yes | 1.01 (0.23 to 4.37) | 0.993 | 1.02 (0.26 to 4.01) | 0.983 |
| Mode of delivery | ||||
| Normal | 1 | 1 | ||
| Caesarean section | 5.18 (1.88 to 14.26) | 0.001 | 5.17 (1.87 to 14.36) | <0.001 |
*Significant confounder adjusted in the multiple log binomial model: mode of delivery.
Sensitivity analysis in both the univariate and multiple log binomial regression models did not change the direction or strength of the estimates compared with the logistic regression model for preterm birth, even though the OR marginally exaggerated the relative risk to some magnitude (table 6).
Table 6.
Sensitivity analysis using log binomial regression for preterm and malaria
| Preterm | Crude RR (CI) | P value | Adjusted RR (CI)* | P value |
| Malaria | ||||
| No | 1 | 1 | ||
| Yes | 1.49 (1.24 to 1.23) | <0.001 | 1.45 (1.20 to 1.78) | <0.001 |
| Parity | ||||
| First pregnancy | 1 | 1 | ||
| 2–3 pregnancies | 0.89 (0.78 to 1.04) | 0.150 | 0.87 (0.74 to 1.02) | 0.087 |
| 4 or more pregnancies | 0.72 (0.57 to 0.91) | 0.006 | 0.74 (0.57 to 0.96) | 0.025 |
| Maternal age (years) | ||||
| 15–24 | 1 | |||
| 25–34 | 1.14 (0.98 to 1.35) | 0.106 | 1.19 (1.01 to 1.42) | 0.039 |
| 35–48 | 0.81 (0.61 to 1.09) | 0.181 | 0.93 (0.67 to 1.29) | 0.670 |
| G6PD | ||||
| Normal | 1 | 1 | ||
| Partial defect | 1.29 (1.03 to 1.60) | 0.025 | 1.27 (1.02 to 1.59) | 0.036 |
| Full defect | 1.04 (0.74 to 1.48) | 0.803 | 0.98 (0.67 to 1.44) | 0.935 |
| Neonatal admission at birth | ||||
| No | 1 | 1 | ||
| Yes | 1.47 (1.13 to 1.91) | 0.004 | 1.39 (1.07 to 1.83) | 0.015 |
*Significant confounders adjusted in the multiple log binomial model: parity, maternal age, G6PD and neonatal admissions at birth.
†RR, Relative Risk
G6PD, glucose-6-phosphate dehydrogenase.
Discussion
In this study, malaria was found in nearly 10% of pregnant women. The study also investigated the associations between malaria and preterm birth, LBW and perinatal mortality.
Prenatal malaria was found to be substantially linked with preterm birth and LBW after correcting for parity, mother’s age, G6PD, SES and neonatal hospitalisation at birth, but not with perinatal death after adjusting for caesarean section. Indeed, Nkwabong et al21 previously reported that third trimester malaria increased the chance of preterm delivery by 5 times and LBW by 2.8 times, which is consistent with our findings. Similarly, Vogel and colleagues22 found that exposure to malaria increased the risk of spontaneous preterm term birth by 1.67 times with a secondary analysis of data from 22 low-income and middle-income countries. van den Broek and colleagues23 similarly conclude from their data that maternal malaria significantly increased the risk of preterm birth by 1.99 times. In Tanzania, similar studies have shown that malaria parasites in mothers’ red blood cells are associated with 3.2 times the risk of premature birth.24 Compared with people without placental malaria, preterm birth rates increased by 4.7% to 5.6% among pregnant women with placental malaria.25 26
In Brazil, researchers reported that P. falciparum species were significantly associated with preterm births, although accounting for less than 40% of the total.27 In contrast, some authors suggest that such correlations are non-significant. For example, a recent comprehensive study of malaria at birth in Uganda that studied three different parasite detection methods, including peripheral and placental blood microscopy, placental blood loop-mediated isothermal amplification (LAMP), and placental histopathology, found no statistically significant link between malaria and preterm birth for any of the methods.28 In this study, it was not possible to distinguish between the various species of malaria. However, previous studies have shown that, regardless of the species, malaria can lead to poor birth outcomes. For example, P. falciparum in placental blood increased the risk of LBW by around 1.7 times in Malawi29 and about 3.7-fold increase in Zaire.30 Furthermore, in Nigeria and Uganda, approximately 33% and 19% of mothers with placental malaria, respectively, delivered LBW babies compared with those without placental malaria.26 31
One interesting aspect that emerged from the analysis is the finding that women who delivered via caesarean section were about five times more likely to suffer perinatal mortality. Notwithstanding, we found that there was no significant increase in the odds of pregnancy mortality in both adjusted and unadjusted models. In contrast, other studies, such as the one conducted in Zaire in 1993, reported that maternal malaria with chloroquine prophylaxis increased the risk of perinatal death by 12 times after adjusting for parity and prenatal clinic visits.30 In addition, P. falciparum malaria during pregnancy increased the risk of neonatal deaths by 2.6 times.32 Infants delivered to mothers with acute placental infections had a fivefold risk of death.33
Multiple variables such as gender, mother’s age and SES can result in adverse birth outcomes. Therefore, in this study, we adjusted for these significant confounders in the multiple logistic regressions and maintained the significant confounders. Women with multiple pregnancies are protected from premature birth. This is apparently due to the extra protection provided by antibodies in subsequent pregnancies against the parasite variant surface antigen VAR2CSA.34 35
Furthermore, regardless of the period of exposure, the effect of malaria exposure on fetal development was detected during the third trimester of pregnancy and has been blamed for poor birth outcomes.6 25 36 This might be because the pathway that connects the mother to the baby throughout pregnancy may impact the fetus’ survival at delivery or even beyond, since the placenta delivers nutrition to the newborn via the umbilical cord. For example, Ouédraogo and his colleagues37 found a connection between umbilical cord parasitaemia and maternal peripheral blood parasitaemia. Also, malaria during pregnancy may have induced excessive stimulation and dysregulated Hb-scavenging system and bioavailability of nitric oxide and L-arginine, which may be associated with poor vascular development and adverse birth outcomes.38 Although we used RDT with peripheral blood, our findings were consistent with the majority of studies on placental malaria.34 35 This is because peripheral blood infections may promote the sequestration of parasites in the placenta and activate immune reactions of antibodies and antigens that may cause complications during delivery.34 35 Furthermore, Kapisi et al25 found that women who had a high burden of malaria had a 14-fold increased risk of placental malaria by blood microscopy and a fourfold increased risk of LAMP. This could indicate that our group had a higher malaria burden, correlating with previous research using a different technique for diagnosis.25
This study benefited from a large sample size. To date, we have not found any studies that examined the relationship between maternal malaria during delivery and the results of birth in Tamale in the Northern Region of Ghana. In this cohort, RDT was used to diagnose malaria, although the sensitivity to malaria was 19% lower than a microscopic examination of peripheral and placenta blood. However, RDT has been reported to have outperformed microscopy in identifying malaria in other settings.36 39 Furthermore, RDT is useful in environments like ours because it produces quick results, requires fewer training and equipment, and has virtually no power interruption. Consequently, especially in hospitals with limited human resources and equipment, rapid malaria detection techniques are imperative for initiating care.
A potential drawback of our study was that we relied on standard laboratories at each hospital to collect data on malaria. While we took every attempt to observe and monitor compliance with established testing techniques, we were unable to account for any measurement bias among laboratory staff for the exposure variable (malaria). However, it should be noted that the RDT method for malaria diagnosis during pregnancy is used routinely in our context and is performed by state-licensed laboratory professionals to diagnose malaria in our population. We also cannot explain the impact of malaria on birth outcomes during the early stages of pregnancy since we only included pregnant women in their third trimester as long as they had done at least one RDT prior to recruitment. This study also failed to account for the number of doses of sulfadoxine/pyrimethamine taken by pregnant women. This denied us the opportunity to measure its confounding effect on malaria and birth outcomes. Nevertheless, our research is comparable with similar studies on the subject matter.
Taken together, these findings indicate that maternal malaria within the third trimester of pregnancy may be a major contributor to LBW and preterm birth in the Northern Region of Ghana.
Supplementary Material
Acknowledgments
We appreciate the Tamale Teaching Hospital and the Ghana Health Service for giving us the opportunity to collect data in their facilities.
Footnotes
Contributors: HH: investigation, writing the draft, data analysis and guarantor. MS: writing, review and editing. MY: conceptualisation and validation. MSH: visualisation and resources. MAS: project administration. PDA: data curation. AF: supervision and acquisition of funds. All authors reviewed and approved the final manuscript.
Funding: This study was supported by the Tehran University of Medical Sciences.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
This study involves human participants and was approved by the ethical review committees of the Ghana Health Service and the Tehran University of Medical Sciences (ethical approval numbers GHS-ERC010/12/17 and IR.TUMS.SPH.REC.1396.4066, respectively). Information sheets with accompanying verbal clarifications were provided to adequately explain all concerns of risk to the study participants. A signed or thumb-printed informed consent was obtained from each participant prior to recruitment. Participants gave informed consent to participate in the study before taking part.
References
- 1.World Health Organization . World malaria report 2021, 2021. [Google Scholar]
- 2.Center for Disease Control and Prevention . Malaria’s Impact Worldwide. Center for Disease Control and Prevention, 2021. [Google Scholar]
- 3.WHO . Protecting malaria high-risk groups, 2021. Available: www.who.int/malaria/areas/high_risk_groups/pregnancy/en/
- 4.WHO . Malaria in pregnancy. In: Guidelines for measuring key monitoring and evaluation indicators (archived), 2007. [Google Scholar]
- 5.WHO . World malaria report 2019. Geneva, 2019. https//www.who.int/publications-detail/world-malaria-report-2019 [Google Scholar]
- 6.Schmiegelow C, Minja D, Oesterholt M, et al. Malaria and fetal growth alterations in the 3(rd) trimester of pregnancy: a longitudinal ultrasound study. PLoS One 2013;8:e53794. 10.1371/journal.pone.0053794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abbas K, Procter SR, van Zandvoort K, et al. Routine childhood immunisation during the COVID-19 pandemic in Africa: a benefit-risk analysis of health benefits versus excess risk of SARS-CoV-2 infection. Lancet Glob Health 2020;8:e1264–72. 10.1016/S2214-109X(20)30308-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Asundep NN, Jolly PE, Carson AP, et al. Effect of malaria and geohelminth infection on birth outcomes in Kumasi, Ghana. Int J Trop Dis Health 2014;4:582–94. 10.9734/IJTDH/2014/7573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ministry of Health . (Ipt) of Malaria in Pregnancy Training Manual for Health Providers Facilitators Guide, 2006. [Google Scholar]
- 10.Ghana PMI. Malaria operational plan FY2018. Ghana MOH Ghana 2018. [Google Scholar]
- 11.UN-Habitat . Ghana: Tamale city profile 2010:1–33.
- 12.Oheneba-Dornyo TV, Amuzu S, Maccagnan A, et al. Estimating the impact of temperature and rainfall on malaria incidence in Ghana from 2012 to 2017. Environ Model Assess 2022;27:473–89. 10.1007/s10666-022-09817-6 [DOI] [Google Scholar]
- 13.Osarfo J, Ampofo GD, Tagbor H. Trends of malaria infection in pregnancy in Ghana over the past two decades: a review. Malar J 2022;21:1–12. 10.1186/s12936-021-04031-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hussein H, Shamsipour M, Yunesian M, et al. Association of adverse birth outcomes with exposure to fuel type use: a prospective cohort study in the Northern region of Ghana. Heliyon 2020;6:e04169. 10.1016/j.heliyon.2020.e04169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hussein H, Shamsipour M, Yunesian M, et al. Fuel type use and risk of respiratory symptoms: a cohort study of infants in the Northern region of Ghana. Sci Total Environ 2021;755:142501. 10.1016/j.scitotenv.2020.142501 [DOI] [PubMed] [Google Scholar]
- 16.Hussein H, Shamsipour M, Yunesian M, et al. Prevalence and predictors of pre-existing hypertension among prenatal women: a cross-sectional study in Ghana. Iran J Public Health 2021;50:1266. 10.18502/ijph.v50i6.6428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehlotra RK, Howes RE, Cramer EY, et al. Plasmodium falciparum Parasitemia and Band Sensitivity of the SD Bioline Malaria Ag P.f/Pan Rapid Diagnostic Test in Madagascar. Am J Trop Med Hyg 2019;100:1196–201. 10.4269/ajtmh.18-1013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ogawa K, Urayama KY, Tanigaki S, et al. Association between very advanced maternal age and adverse pregnancy outcomes: a cross sectional Japanese study. BMC Pregnancy Childbirth 2017;17:1–10. 10.1186/s12884-017-1540-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lisonkova S, Janssen PA, Sheps SB, et al. The effect of maternal age on adverse birth outcomes: does parity matter? Journal of Obstetrics and Gynaecology Canada 2010;32:541–8. 10.1016/S1701-2163(16)34522-4 [DOI] [PubMed] [Google Scholar]
- 20.Bennett DA. How can I deal with missing data in my study? Aust N Z J Public Health 2001;25:464–9. 10.1111/j.1467-842X.2001.tb00294.x [DOI] [PubMed] [Google Scholar]
- 21.Nkwabong E, Mayane DN, Meka E, et al. Malaria in the third trimester and maternal‐perinatal outcome. Int J Gynecol Obstet 2020;151:103–8. 10.1002/ijgo.13261 [DOI] [PubMed] [Google Scholar]
- 22.Vogel JP, Lee ACC, Souza JP. Maternal morbidity and preterm birth in 22 low- and middle-income countries: a secondary analysis of the who global survey dataset. BMC Pregnancy Childbirth 2014;14:56. 10.1186/1471-2393-14-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van den Broek NR, Jean-Baptiste R, Neilson JP. Factors associated with preterm, early preterm and late preterm birth in Malawi. PLoS One 2014;9:e90128. 10.1371/journal.pone.0090128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Menendez C, Ordi J, Ismail MR, et al. The impact of placental malaria on gestational age and birth weight. J Infect Dis 2000;181:1740–5. 10.1086/315449 [DOI] [PubMed] [Google Scholar]
- 25.Kapisi J, Kakuru A, Jagannathan P, et al. Relationships between infection with Plasmodium falciparum during pregnancy, measures of placental malaria, and adverse birth outcomes. Malar J 2017;16:400. 10.1186/s12936-017-2040-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Washburn EE, Kakuru A, Muhindo MK, et al. 865: Malaria exposure in pregnancy: birth outcomes with placental and non-placental malaria infection. Am J Obstet Gynecol 2018;218:S515–6. 10.1016/j.ajog.2017.11.402 [DOI] [Google Scholar]
- 27.Dombrowski JG, de Souza RM, Silva NRM, et al. Malaria during pregnancy and newborn outcome in an unstable transmission area in Brazil: a population-based record linkage study. PLoS One 2018;13:e0199415. 10.1371/journal.pone.0199415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ategeka J, Kakuru A, Kajubi R, et al. Relationships between measures of malaria at delivery and adverse birth outcomes in a high-transmission area of Uganda. J Infect Dis 2020;222:863–70. 10.1093/infdis/jiaa156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steketee RW, Wirima JJ, Hightower AW, et al. The effect of malaria and malaria prevention in pregnancy on offspring birthweight, prematurity, and intrauterine growth retardation in rural Malawi. Am J Trop Med Hyg 1996;55:33–41. 10.4269/ajtmh.1996.55.33 [DOI] [PubMed] [Google Scholar]
- 30.Nyirjesy P, Kavasya T, Axelrod P, et al. Malaria during pregnancy: neonatal morbidity and mortality and the efficacy of chloroquine chemoprophylaxis. Clin Infect Dis 1993;16:127–32. 10.1093/clinids/16.1.127 [DOI] [PubMed] [Google Scholar]
- 31.Oraneli BU, Okeke OC, Ubachukwu PO. Effect of placental malaria on birth weight of babies in Nnewi, Anambra state, Nigeria. J Vector Borne Dis 2013;50:13. [PubMed] [Google Scholar]
- 32.Moore KA, Fowkes FJI, Wiladphaingern J, et al. Mediation of the effect of malaria in pregnancy on stillbirth and neonatal death in an area of low transmission: observational data analysis. BMC Med 2017;15:1–11. 10.1186/s12916-017-0863-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bardají A, Sigauque B, Sanz S, et al. Impact of malaria at the end of pregnancy on infant mortality and morbidity. J Infect Dis 2011;203:691–9. 10.1093/infdis/jiq049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cutts JC, Agius PA. Pregnancy-Specific malarial immunity and risk of malaria in pregnancy and adverse birth outcomes: a systematic review. BMC Med 2020;18:1–21. 10.1186/s12916-019-1467-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Babakhanyan A, Fang R, Wey A, et al. Comparison of the specificity of antibodies to VAR2CSA in Cameroonian multigravidae with and without placental malaria: a retrospective case–control study. Malar J 2015;14:1–10. 10.1186/s12936-015-1023-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kattenberg JH, Ochodo EA, Boer KR, et al. Systematic review and meta-analysis: rapid diagnostic tests versus placental histology, microscopy and PCR for malaria in pregnant women. Malar J 2011;10:321. 10.1186/1475-2875-10-321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ouédraogo A, Tiono AB, Diarra A, et al. Transplacental transmission of Plasmodium falciparum in a highly malaria endemic area of Burkina Faso. J Trop Med 2012;2012:1–7. 10.1155/2012/109705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ngai M, Weckman AM, Erice C, et al. Malaria in pregnancy and adverse birth outcomes: new mechanisms and therapeutic opportunities. Trends Parasitol 2020;36:127–37. 10.1016/j.pt.2019.12.005 [DOI] [PubMed] [Google Scholar]
- 39.Minja DTR, Schmiegelow C, Oesterholt M, et al. Reliability of rapid diagnostic tests in diagnosing pregnancy-associated malaria in north-eastern Tanzania. Malar J 2012;11:1–10. 10.1186/1475-2875-11-211 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request.