Abstract
Regular insulin can reduce hyperglycemia when directly added to total parenteral nutrition (TPN) solutions. Insulin is not routinely added to all TPN solutions. For patients who require insulin prior to the initiation of TPN supplement, one-third to one-half of the usual total daily dose can be added to the TPN bag as regular human insulin. However, an incorrect dose or an interaction between insulin and the TPN bag material may affect blood sugar control in clinical practice. Therefore, it is important to quantitatively determine the final dose of insulin in the TPN bag. High performance liquid chromatography is a very powerful technique for determining the purity of proteins. The goal of this study was to use high-performance liquid chromatography to perform quantitative analysis of insulin in a TPN bag. The analysis was performed under different light conditions (UV, fluorescent, and darkness) and different temperatures (25°C and 2–8°C). The results show that adsorption of insulin on an ethylene vinyl acetate TPN bag is significantly higher than that on glass. Based on the results, it is evident that regular insulin should be administered separately from TPN to reduce cost and eliminate wasteful disposal of TPN solutions.
Keywords: adsorption insulin total parenteral nutrition bags
1. Introduction
Traditionally, people who are unable to eat on their own are tube fed via the mouth. This method of delivering nutrients directly to the stomach is referred to as enteral nutrition. In 1968, Dudrick et al [1] investigated the delivery of small volumes of highly concentrated nutrients through the superior vena cava (fast blood flow, concentrated nutrients diluted by blood). Since then, a new form of acquiring nutrients, called total parenteral nutrition (TPN), has been introduced [2]. Previous studies have shown that up to 50% of hospitalized patients are malnourished. Supplemental nutrients help patients deal with stress and can mitigate the complications associated with diseases and treatments, e.g., infection and surgery [3–5]. Thus, the importance of supplementary nutrition for patients under stress is obvious.
TPN primarily consists of nutrients, micronutrients, and other individual components. Insulin is often included in TPN to control blood sugar levels because hyperglycemia is the most common complication associated with TPN. The occurrence of hyperglycemia in patients receiving TPN can be as high as 47%. Typically, insulin is administered to control blood sugar levels and achieve euglycemia. Stable euglycemia reduces the complications associated with blood sugar monitoring and insulin administration, thus reducing the burden on health care providers [6–8].
Regular insulin can reduce hyperglycemia when it is directly added to the TPN solution [9]. However, hypoglycemia may occur when insulin is continually administered. Hypoglycemia is less likely to result from a decrease in the fluid rate or inadvertent interruption of TPN fluid if insulin is added directly to the fluid rather than given by subcutaneous injection [9,10]. Insulin is not routinely added to all TPN solutions. For patients who require insulin prior to the initiation of TPN, one-third to one-half of the usual total daily dose can be added to the TPN bag as regular human insulin. Depending on blood glucose levels, additional subcutaneous insulin may be administered. Thus, precise dosages of insulin in the TPN solution can help health-care providers control blood sugar levels better. However, under- or overestimated doses of insulin in the TPN bag, or interactions between insulin and the TPN bag material may affect blood sugar control significantly. Therefore, it is important to quantitatively determine the precise dose of insulin to be added to the TPN solution.
In 1951, Ferrebee et al [11] suggested that insulin can be adsorbed on a glass infusion container. Since then, insulin has been found to be adsorbed on polyvinylchloride, polyethylene, and silicone-treated glassware infusion sets. Many factors such as purity and source of TPN solution, TPN package material, and insulin itself have changed over the past decades. For example, the purity of insulin has increased by the use of recombinant DNA technology to produce human insulin. The TPN package is now made of nonpolyvinylchloride material such as ethylene vinyl acetate (EVA).
Concentration analyses of insulin in the TPN solution have revealed several factors including the effect of the infusion solution, packaging materials, temperature, and light exposure on insulin [12–14]. From a previous research, insulin was tagged with I125 and then added to an EVA bag containing amino acid, dextrose, electrolytes, heparin, and vitamin. The availability of human insulin was higher than that reported in the literature [14]. However, this research also found that the amount of insulin that binds to the infusion material was much greater than the amount that binds to the components in the TPN solution. Adsorption was related to the surface area of the bag. For example, if the same amount of insulin were added to bags with different volumes (500 mL and 1000 mL), adsorption would be greater for bags with greater volumes [14]. In addition, a previous research also indicated that different packaging materials had different insulin adsorption capacities. Soda and silicon-soda glass materials, as compared to plastic materials, have higher insulin adsorption capacity. Sodium chloride, potassium chloride, and water-soluble vitamins do not seem to affect insulin adsorption on these materials [12]. Furthermore, infusion tubing materials can contribute to insulin adsorption. In a study, it was observed that a glass container resulted in 52% insulin adsorption and that plastic tubing contributed another 55.4%, resulting in total insulin adsorption of >78.8% [13].
The effect of temperature on insulin adsorption in the TPN solution is subtle. Research on the effect of temperature on insulin adsorption in a dialysis bag indicated that regular insulin shows greater adsorption at a high temperature (37°C) than at a low temperature (24°C); however, adsorption capacity decreases as the insulin dosage increases [15]. To the best of our knowledge, no study has addressed the effect of light on insulin adsorption in a TPN bag. Most TPN finished products are sealed with a tin foil after preparation and, thus, are not exposed to light. Unfortunately, once sealed, it becomes difficult to check TPN end products for impurities and identify insulin levels of the patient in clinical practice. This sealing method is currently not implemented in our institution.
Many nonimmune and immune methods have been reported for the determination of insulin in in vivo or in vitro media, including radioimmunoassay [16], enzyme immunoassay [17,18], capillary electrophoresis [19], luminescent immunoassay [20], and high performance liquid chromatography (HPLC) [21–24]. HPLC has widely been used for human insulin detection since 1986 and has been applied to quantitative analyses of insulin in various preparations [25].
Despite various factors, such as container material, light exposure time, and temperature, which have been reported to possibly affect insulin in TPN, in Taiwan, studies to quantitatively determine the final dose of insulin in a TPN bag are limited. Therefore, the goal of this experiment is to determine whether the insulin in a TPN bag is affected by the package material, light exposure time, and temperature, using HPLC analysis.
2. Methods
In this study, we primarily used HPLC to analyze the insulin solution for injection into an EVA TPN bag. Quantitative analyses of insulin were performed under different temperature and light exposure conditions.
2.1. Insulin samples
Several insulin products used in our hospital were evaluated and compared. Rapid-acting clear insulin Actrapid HM® (1000 U/10 mL/vial) (Novo Nordisk A/S/Kalundborg, Denmark) was selected for this study. Prior to performing the quantitative analysis, each sample of rapid-acting clear insulin Actrapid HM (1000 U/10 mL/vial) was maintained at 2–8°C.
Fifty international units of Actrapid were added to a TPN bag preloaded with 50 mL of water. A 500 mL Baxter EVA TPN bag was used in this study. Fifty international units of insulin were placed in a general glass tube. The procedure was checked by two pharmacists (an operator and a monitor). The contents were thoroughly mixed, and after different incubation times, quantitative analysis of the insulin was performed using HPLC.
2.2. Study procedures
In this study, we used a UV spectrophotometer to scan the insulin absorption spectrum to determine the appropriate wavelength. This allowed us to establish the insulin standard curve for Actrapid. Then, we tested intra- and interday precision.
We placed the TPN bag and glass tube in different temperature environments, including room temperature (25°C), and under refrigeration (2–8°C). We performed HPLC quantitative analysis with different sampling times (0 hours, 0.25 hours, 0.5 hours, 1 hour, 2 hours, 4 hours, 8 hours, 16 hours, 24 hours, 36 hours, 48 hours, and 72 hours). In addition, we prepared the EVA TPN bag and glass tube in different illumination environments, including a fluorescent tube and a UV lamp. As before, we performed HPLC quantitative analysis with sampling times of 0 hours, 0.25 hours, 0.5 hours, 1 hour, 2 hours, 4 hours, 8 hours, 16 hours, 24 hours, 36 hours, 48 hours, and 72 hours.
2.3. Chemicals and equipment
We used the Agilent 1100 Series HPLC System, an Agilent 8453 UV spectrophotometer, and an Intersil ODS-2 C18 column (5 μm, 150 mm × 4.6 mm) (GL Science/Shinjuku-ku, Tokyo, Japan). HPLC-grade methanol was purchased from Merck (Darmstadt, Germany), HPLC-grade acetonitrile was purchased from Fisher (Waltham, Massachusetts, USA), analytical-grade trifluoroacetic acid (>99%) was purchased from Acros (Geel, Belgium), and injection water was purchased from Nang Kuang Pharmaceuticals (Tainan city, Taiwan).
The sample withdrawn from the TPN bag was then analyzed using an HPLC method (wavelength 214 nm; pressure 102 bar) modified from a previous study [26]. The mobile phase was a mixture of acetonitrile, water, and trifluoroacetic acid in the ratio 500:500:1. The flow, run time, and sample volume were 1 mL/min, 10 minutes, and 20 μL, respectively. Prior to the experiment, the mobile phase was degassed for 30 minutes. At the end of the experiment, the column was washed with methanol for 30 minutes and with water for another 30 minutes. We used 0.1700 g of methylparaben dissolved in 2.0 L of 0.01M HCl as the internal standard. Each data point represented the average of three determinations.
2.4. Outcomes
TPN products were observed under different temperature (25°C and 2–8°C) and light exposure (UV, fluorescent, and darkness) conditions. The percentage of insulin adsorption was calculated using mAU of sampling time 0 as the denominator and mAU of different sampling times as the numerator. The percentage change of insulin adsorption was plotted and compared with that of the control group (glass material).
2.5. Statistical analysis
We used analysis of variance to analyze differences in percentage adsorption of insulin at different times under different conditions, including different materials (TPN bag or glass), different temperatures (25°C and 2–8°C), and different light exposure conditions (UV, fluorescent, and darkness). Statistical significance was defined as a two-sided p value of <0.05. All statistical analyses were performed using Software package SPSS version 18.0 (SPSS Inc., Chicago, IL, USA).
3. Results and discussion
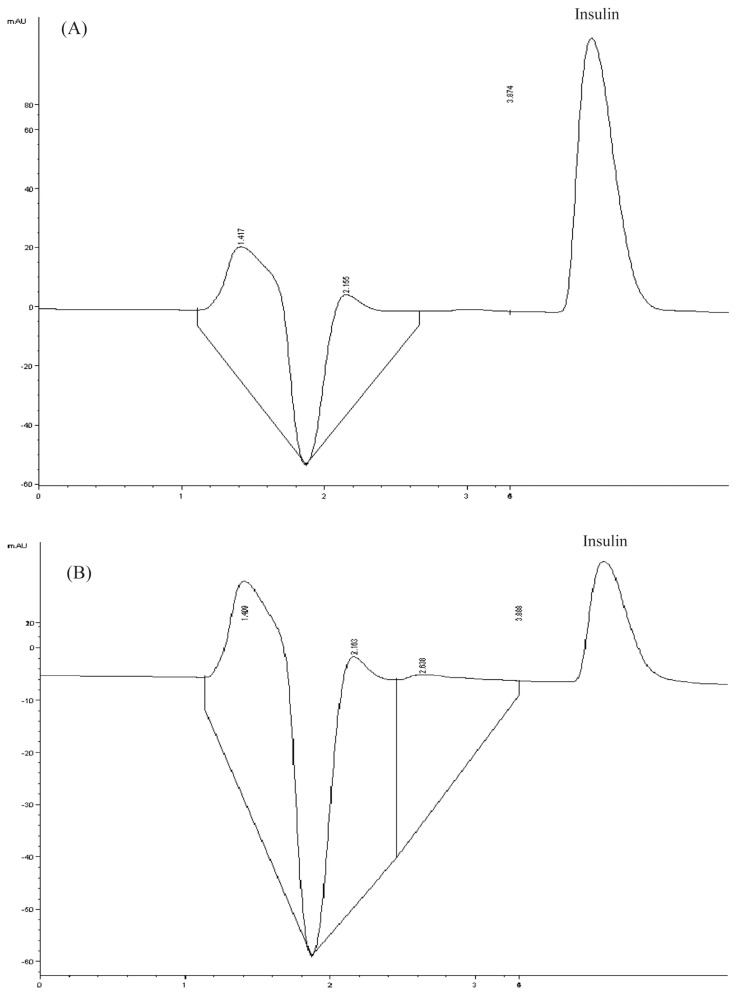
Clinically, if the TPN solution was to be used over the weekend, the solution would be prepared 1 day earlier and stored under refrigeration (2–8°C). In this study, the known factors (variables) affecting insulin adsorption in the TPN solution were temperature, time, and light exposure. The present study investigated the effect of the container material on insulin adsorption. We found that for all variables, the EVA TPN bag used by our institution had a greater effect on insulin adsorption than the glass container. The HPLC profiles of insulin in glass and TPN bag at 25°C and under darkness are shown in Fig. 1. The HPLC profiles of the two materials were strikingly different. The result showed that the glass container did not adsorb insulin (Fig. 1A) but the TPN bag did (Fig. 1B).
Fig. 1.
(A) Chromatogram of insulin in glass at 25°C and under darkness. (B) Chromatogram of insulin in a plastic bag at 25°C and under darkness.
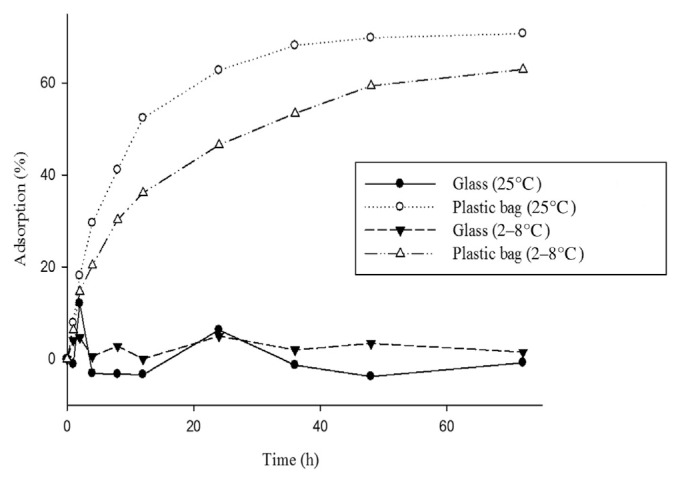
3.1. Temperature and container material
Fig. 2 illustrates the positive correlation of time to insulin adsorption on the EVA bag. The same results were observed under both room temperature and refrigerated conditions. In addition, for the same conditions, absorption by the plastic bag was greater than that by the glass container. At 25°C, without light exposure for 24 hours, insulin adsorption on the EVA bag was 62.71% and that on the glass material was 6.34%, with a significant p value of <0.05.
Fig. 2.
Adsorption of insulin on different materials and at different temperatures under darkness: glass compared to plastic bag at 25°C, p < 0.001; glass compared to plastic bag at 2–8°C, p < 0.001.
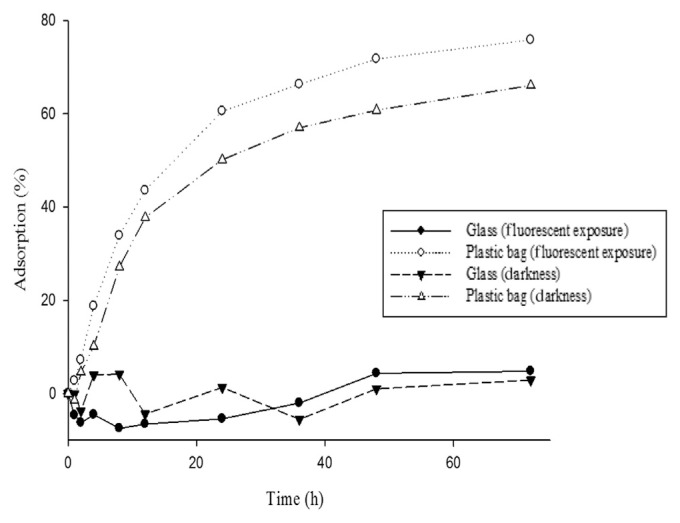
3.2. Light exposure
Under darkness, insulin adsorption on the EVA bag at 25°C was 62.71%. Although the value is greater than that for refrigerated adsorption (46.51%), the difference is not statistically significant. As shown in Fig. 2, adsorption reached the maximum value after 36 hours.
Under the same temperature, but different light exposure conditions (Fig. 3), adsorption on the plastic bag was greater than that on the glass container. At 25°C, insulin adsorption on the EVA bag was 60.52%, whereas that on the glass material was −5.43%. The p value was <0.05, which is statistically significant. Similar results were observed at 25°C under darkness. However, if the same materials were used, the light exposure difference would have been statistically insignificant.
Fig. 3.
Adsorption of insulin on different materials and under light exposure condition at 25°C: glass exposed to a fluorescent light compared to plastic bag exposed to a fluorescent light, p = 0.001; glass under darkness compared to plastic bag under darkness, p = 0.005.
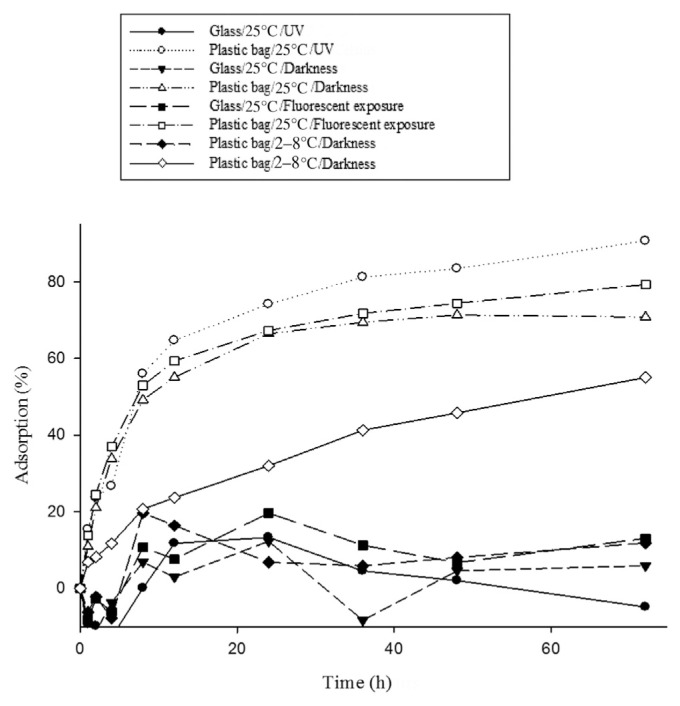
3.3. Light sources
Under different light sources, insulin adsorption on the EVA bag is still greater than that on the glass material (Fig. 4). At 25°C, under UV light exposure, insulin adsorption on the EVA bag and that on the glass material was 74.12% and 13.34%, respectively. At 25°C, under darkness, insulin adsorption on the EVA bag and that on the glass material was 66.41% and 12.31%, respectively. The p values were <0.05 and were thus statistically significant.
Fig. 4.
Adsorption of insulin on different materials, at different temperatures, and under light exposure condition: glass exposed to UV light compared to plastic bag at 25°C, p < 0.001; glass compared to plastic bag at 25°C and under darkness, p < 0.001; glass compared to plastic bag at 25°C and under exposure to fluorescent light, p < 0.001; glass compared to plastic bag at 2–8°C and under darkness, p = 0.004.
Insulin adsorption on infusion materials can be prevented or reduced by serum albumin. In addition, heparin and dextran have been reported to reduce insulin adsorption but with subtle results. Albumin reduction of insulin adsorption has the greatest effect at a concentration of approximately 0.1%. However, the addition of albumin has economic and potential contamination issues; consequently, it is not implemented at our institution.
Clinically, insulin administration can be categorized into the following methods: (1) insulin administration in a TPN solution; (2) direct intramuscular administration; (3) coadministration with TPN and intramuscular methods, and (4) continuous insulin infusion. When insulin is administrated in TPN, the insulin dose must be modified after the blood sugar level has been stabilized, in order to prevent hypoglycemia. When this occurs, it is possible that all of the prepared TPN will be discarded. A previous study has indicated that separate insulin infusion can save 7.3 L of TPN solution per patient, which represents a saving of approximately $395. Patients utilizing separate insulin infusion can have their blood sugar level maintained at approximately 100–250 mg/dL in ~73% of 636 blood draws; blood sugar levels never fall below 50 mg/dL. Benefits of separate insulin infusion also include reduction of catheter sepsis.
3.4. Conclusion
In this study, HPLC was used to perform quantitative analysis of insulin adsorption on different materials, i.e., EVP TPN bag and glass, under different light and temperature conditions. The results indicate a significant difference (p < 0.05) in the adsorption rates. Adsorption of insulin on EVA bags was greater than that on glass under different light (UV, fluorescent, and darkness) and temperature (25°C and 2–8°C) conditions. Thus, it is reasonable to conclude that regular insulin should be administered separately from TPN to reduce cost and eliminate wasteful disposal of TPN solutions.
Acknowledgments
This study was supported by a grant from the Kaohsiung Medical University Hospital, Kaohsiung, Taiwan (93-ND-040) and by Kaohsiung Medical University Hospital, Kaohsiung, Taiwan (KMUH103-3R69). The authors would like to thank Enago (www.enago.tw) for the English language review. The authors would like to thank Chai-Lin Kao at the Department of Medicinal and Applied Chemistry, Kaohsiung Medical University, for assistance with HPLC work.
Funding Statement
This study was supported by a grant from the Kaohsiung Medical University Hospital, Kaohsiung, Taiwan (93-ND-040) and by Kaohsiung Medical University Hospital, Kaohsiung, Taiwan (KMUH103-3R69).
Footnotes
Conflicts of interest
All contributing authors declare no conflicts of interest.
REFERENCES
- 1. Dudrick SJ, Wilmore DW, Vars HM, Rhoads JE. Long-term total parenteral nutrition with growth, development, and positive nitrogen balance. Surgery. 1968;64:134–42. [PubMed] [Google Scholar]
- 2. Wilmore DW, Groff DB, Bishop HC, Dudrick SJ. Total parenteral nutrition in infants with catastrophic gastrointestinal anomalies. J Pediatr Surg. 1969;4:181–9. doi: 10.1016/0022-3468(69)90389-3. [DOI] [PubMed] [Google Scholar]
- 3. Shiroma GM, Horie LM, Castro MG, Martins JR, Bittencourt AF, Logullo L, Teixeira da Silva Mde L, Waitzberg DL. Nutrition quality control in the prescription and administration of parenteral nutrition therapy for hospitalized patients. Nutr Clin Pract. 2015;30:406–13. doi: 10.1177/0884533614567540. [DOI] [PubMed] [Google Scholar]
- 4. Dumlu EG, Özdedeoğlu M, Bozkurt B, Tokaç M, Yalçin A, Öztürk L, Kiliç M. A general consideration of the importance of nutrition for critically ill patients. Turk J Med Sci. 2014;44:1055–9. doi: 10.3906/sag-1308-68. [DOI] [PubMed] [Google Scholar]
- 5. Patel V, Romano M, Corkins MR, DiMaria-Ghalili RA, Earthman C, Malone A, Miller S, Sabino K, Wooley J, Guenter P. Nutrition screening and assessment in hospitalized patients: a survey of current practice in the United States. Nutr Clin Pract. 2014;29:483–90. doi: 10.1177/0884533614535446. [DOI] [PubMed] [Google Scholar]
- 6. Olveira G, Tapia MJ, Ocón J, Cabrejas-Gómez C, Ballesteros-Pomar MD, Vidal-Casariego A, Arraiza-Irigoyen C, Olivares J, Conde-García Mdel C, García-Manzanares A, Botella-Romero F, Quílez-Toboso RP, Cabrerizo L, Matia P, Chicharro L, Burgos R, Pujante P, Ferrer M, Zugasti A, Prieto J, Diéguez M, Carrera MJ, Vila-Bundo A, Urgelés JR, Aragón-Valera C, Rovira A, Bretón I, García-Peris P, Muñoz-Garach A, Márquez E, Del Olmo D, Pereira JL, Tous MC. Parenteral nutrition-associated hyperglycemia in non-critically ill inpatients increases the risk of in-hospital mortality (multicenter study) Diabetes Care. 2013;36:1061–6. doi: 10.2337/dc12-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Alaedeen DI, Walsh MC, Chwals WJ. Total parenteral nutrition-associated hyperglycemia correlates with prolonged mechanical ventilation and hospital stay in septic infants. J Pediatr Surg. 2006;41:239–44. doi: 10.1016/j.jpedsurg.2005.10.045. [DOI] [PubMed] [Google Scholar]
- 8. Cheung NW, Napier B, Zaccaria C, Fletcher JP. Hyperglycemia is associated with adverse outcomes in patients receiving total parenteral nutrition. Diabetes Care. 2005;28:2367–71. doi: 10.2337/diacare.28.10.2367. [DOI] [PubMed] [Google Scholar]
- 9. Knapke CM, Owens JP, Mirtallo JM. Management of glucose abnormalities in patients receiving total parenteral nutrition. Clin Pharm. 1989;8:136–44. [PubMed] [Google Scholar]
- 10. Olveira G, Tapia MJ, Ocón J, Cabrejas-Gómez C, Ballesteros-Pomar MD, Vidal-Casariego A, Arraiza-Irigoyen C, Olivares J, Conde-García MC, García-Manzanares Á, Botella-Romero F, Quílez-Toboso RP, Matía P, Rubio MÁ, Chicharro L, Burgos R, Pujante P, Ferrer M, Zugasti A, Petrina E, Manjón L, Diéguez M, Carrera MJ, Vila-Bundo A, Urgelés JR, Aragón-Valera C, Sánchez-Vilar O, Bretón I, García-Peris P, Muñoz-Garach A, Márquez E, Del Olmo D, Pereira JL, Tous MC. Hypoglycemia in noncritically ill patients receiving total parenteral nutrition: a multicenter study. (Study group on the problem of hyperglycemia in parenteral nutrition; Nutrition area of the Spanish Society of Endocrinology and Nutrition.) Nutrition. 2015;31:58–63. doi: 10.1016/j.nut.2014.04.023. [DOI] [PubMed] [Google Scholar]
- 11. Ferrebee JW, Johnson BB, Mithoefer JC, Gardella JW. Insulin and adrenocorticotropin labelled with radio-iodine. Endocrinology. 1951;48:277–83. doi: 10.1210/endo-48-3-277. [DOI] [PubMed] [Google Scholar]
- 12. Schildt B, Ahlgren T, Berghem L, Wendt Y. Adsorption of insulin by infusion materials. Acta Anaesthesiol Scand. 1978;22:556–62. doi: 10.1111/j.1399-6576.1978.tb01338.x. [DOI] [PubMed] [Google Scholar]
- 13. Petty C, Cunningham NL. Insulin adsorption by glass infusion bottles, polyvinylchloride infusion containers, and intravenous tubing. Anesthesiology. 1974;40:400–4. doi: 10.1097/00000542-197404000-00018. [DOI] [PubMed] [Google Scholar]
- 14. Marcuard SP, Dunham B, Hobbs A, Caro JF. Availability of insulin from total parenteral nutrition solutions. JPEN J Parenter Enteral Nutr. 1990;14:262–4. doi: 10.1177/0148607190014003262. [DOI] [PubMed] [Google Scholar]
- 15. Twardowski ZJ, Nolph KD, McGary TJ, Moore HL. Influence of temperature and time on insulin adsorption to plastic bags. Am J Hosp Pharm. 1983;40:583–6. [PubMed] [Google Scholar]
- 16. Dezier JF, Jouanolle AM, Le Reun M, Poirier JY. Comparison of 2 methods of measuring microalbuminuria. Immunonephelometry and radioimmunology. Ann Biol Clin (Paris) 1987;45:78–84. [PubMed] [Google Scholar]
- 17. Shen H, Aspinwall CA, Kennedy RT. Dual microcolumn immunoassay applied to determination of insulin secretion from single islets of Langerhans and insulin in serum. J Chromatogr B Biomed Sci Appl. 1997;689:295–303. doi: 10.1016/s0378-4347(96)00336-2. [DOI] [PubMed] [Google Scholar]
- 18. Zaitsu K, Kimura Y, Ohba Y, Hamase K, Motomura Y, Itose M, Ishiyama M. Hemeundecapeptide labeling on insulin for the immunoassay of insulin with chemiluminescence detection. Anal Sci. 1999;15:871–8. [Google Scholar]
- 19. German I, Kennedy TR. Rapid simultaneous determination of glucagon and insulin by capillary electrophoresis immunoassays. J Chromatogr B Biomed Sci Appl. 2000;742:353–62. doi: 10.1016/s0378-4347(00)00180-8. [DOI] [PubMed] [Google Scholar]
- 20. Arcelloni C, Falqui L, Martinenghi S, Pontiroli AE, Paroni R. Capillary electrophoresis for simultaneous quantification of human proinsulin, insulin and intermediate forms. Electrophoresis. 1998;19:1475–7. doi: 10.1002/elps.1150190843. [DOI] [PubMed] [Google Scholar]
- 21. Hvass A, Skelbaek-Pedersen B. Determination of protamine peptides in insulin drug products using reversed phase high performance liquid chromatography. J Pharm Biomed Anal. 2005;37:551–7. doi: 10.1016/j.jpba.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 22. Toriumi C, Imai K. Determination of insulin in a single islet of Langerhans by high performance liquid chromatography with fluorescence detection. Anal Chem. 2002;74:2321–7. doi: 10.1021/ac020043t. [DOI] [PubMed] [Google Scholar]
- 23. Pastore A, Bernardini S, DelloStrologo L, Rizzoni G, Cortese C, Federici G. Simultaneous determination of insulin and p-aminohippuric acid in plasma and urine by reversed-phase high performance liquid chromatography. J Chromatogr B. 2011;751:187–91. doi: 10.1016/s0378-4347(00)00444-8. [DOI] [PubMed] [Google Scholar]
- 24. Jars MU, Hvass A, Waaben D. Insulin aspart (AspB28 human insulin) derivatives formed in pharmaceutical solutions. Pharm Res. 2002;19:621–8. doi: 10.1023/a:1015302012070. [DOI] [PubMed] [Google Scholar]
- 25. Fisher BV, Smith D. HPLC as a replacement for the animal response assays for Insulin. J Pharm Biomed Anal. 1986;4:377–87. doi: 10.1016/0731-7085(86)80059-0. [DOI] [PubMed] [Google Scholar]
- 26. Abu Heshmeh N, Sallam A, Mater Y, Alawi M. A new method for determination of human insulin in aqueous injections. Int J Pharm Biomed Res. 2013;4:428–35. [Google Scholar]