Abstract
Because of the high incidence of cardiovascular diseases in Asian countries, traditional fermented foods from Asia have been increasingly investigated for antiatherosclerotic effects. This study investigated the production of nattokinase, a serine fibrinolytic enzyme, in pigeon pea by Bacillus subtilis fermentation. B. subtilis 14714, B. subtilis 14715, B. subtilis 14716, and B. subtilis 14718 were employed to produce nattokinase. The highest nattokinase activity in pigeon pea was obtained using B. subtilis 14715 fermentation for 32 hours. In addition, the levels of antioxidants (phenolics and flavonoids) and angiotensin converting enzyme inhibitory activity were increased in B. subtilis 14715-fermented pigeon pea, compared with those in nonfermented pigeon pea. In an animal model, we found that both water extracts of pigeon pea (100 mg/kg body weight) and water extracts of B. subtilis-fermented pigeon pea (100 mg/kg body weight) significantly improved systolic blood pressure (21 mmHg) and diastolic blood pressure (30 mmHg) in spontaneously hypertensive rats. These results suggest that Bacillus-fermented pigeon pea has benefits for cardiovascular health and can be developed as a new dietary supplement or functional food that prevents hypertension.
Keywords: antioxidants, Bacillus subtilis, fermentation, nattokinase, pigeon pea
1. Introduction
Atherosclerosis is the basis of coronary artery disease and one of the most serious cardiovascular diseases (CVDs) in man. The chronic injuries of blood vessels that are characteristics of this disease lead to thrombotic embolism [1]. Consequently, a great deal of effort has been expended in preventing atherosclerosis and the resulting thrombotic diseases [2]. In recent years, many studies have analyzed the influence of dietary habits on the generation and mitigation of atherosclerosis [1]. Because of the low incidence of CVDs in Asian countries, traditional fermented foods from Asia have been the subject of medical research [3]. Thus, it has been suggested that fermented and pickled foods, including fruits, vegetables, and cereals, may be beneficial for their nutrient contents, such as minerals, vitamins, dietary fiber, oligosaccharides, phytosterols, and antioxidants [4].
During the fermentation of foods, desirable biochemical changes and significant modifications in flavor and texture are brought about through the activity of microorganisms or enzymes. For instance, extracts of natto, a traditional food made by the fermentation of cooked soybean by Bacillus subtilis, are known to consist of nattokinase, a serine fibrinolytic enzyme, that is secreted from vegetative B. subtilis cells and which has strong fibrinolytic activity [5]. The in vivo anticoagulant activity of nattokinase enhances blood circulation by dissolving fibrin and soluble fibrin monomers. Because this kinase reduces blood clotting, it helps to prevent CVDs [6]. Recent studies have demonstrated that many of the ingredients of natto are useful in the prevention of CVD and hypertension [7].
Pigeon pea (Cajanus cajan L.) is an important grain legume crop of rain-field agriculture in the tropics and subtropics. Pigeon pea is a food that contains high levels of phytosterol, which has been shown to possess antidyslipidemic activity [8]. Although utilization of red gram for human nutrition may be constrained by the presence of protease inhibitors [9], the extracts of pigeon pea are commonly used to treat diabetes, febrifuge, dysentery, hepatitis, measles, sickle cell anemia, and hepatic disorders all over the world [10,11]. Recently, we have found that pigeon pea may attenuate inflammation and avoid oxidative damage in RAW264.7 macrophages [12]. The purpose of this study was to investigate the antihypertensive properties of nattokinase, angiotensin converting enzyme inhibition (ACEI) activity, and antioxidative activities from pigeon pea fermented by B. subtilis.
2. Methods
2.1. Bacterial strain and pigeon pea fermentation
Pigeon pea was provided from Taitung District Agricultural Research and Extension Station of Council of Agriculture (Taitung, Taiwan). The strains of B. subtilis 14714, B. subtilis 14715, B. subtilis 14716, and B. subtilis 14718 were purchased from Bioresource Collection and Research Center, Hsinchu, Taiwan. The strains were cultured in nutrient broth [6] and incubated at 37°C for 24 hours. Each activated culture was then inoculated into 10 mL of nutrient broth and incubated at 37°C for 16 hours. The cultures were subsequently diluted to 108–109 colony forming units/mL and used to inoculate steamed pigeon pea for fermentation. Briefly, 25 g of pigeon pea was washed and soaked in distilled water for 18 hours. After decanting the water, the pigeon pea was placed on a shelf and steamed at 121°C for 30 minutes. The steamed pigeon pea was cooled, and inoculated with bacterial inoculum (2%) and incubated at various temperatures (30°C, 35°C, 37°C, 40°C, and 45°C) and various relative humidities (75%, 80%, 85%, 90%, and 95%) for various times (8 hours, 16 hours, 24 hours, 32 hours, 40 hours, and 48 hours) to evaluate the optimal conditions for nattokinase and ACEI production. At the end of fermentation, all samples were freeze-dried (Alpha 1-2/LD-2 freeze-dryer, RZ-5 vacuum pump, Christ, Osterode am Harz, Germany). Subsequently, the powder of B. subtilis-fermented pigeon pea was extracted with distilled water (1:10/v:v), and the extract was freeze-dried and stored at −20°C.
2.2. Assay for ACEI activity of B. subtilis-fermented pigeon pea
A 45 μL sample of B. subtilis-fermented pigeon pea extract was added to 45 μL of ACE (final concentration: 33 mU/mL) previously dissolved in 0.1M sodium-borate buffer (pH 8.3), and the mixture was allowed to react for 10 minutes at 37°C. Subsequently, 45 μL of 3mM N-hippuryl-l-histidyl-l-leucine hydrate was added and incubated at 37°C for 30 minutes, and this reaction was stopped with 150 μL of 0.1N hydrochloric acid. Twenty microliters of reacted solution was analyzed using high-performance liquid chromatography (PU2089 plus, Jasco Co., Tokyo, Japan). Chromatographic separation was conducted on a C18 column (25 cm × 4.6 mm internal diameter, 5 μm, Discovery Bellefonte, PA, USA). An acetonitrile/water (0:100–70:30 with 0.05% trifluoroacetic acid) solution was used as the mobile phase, and the flow rate was 1 mL/min. The ACEI activity was compared with hippuric acid synthesis using an ultraviolet detector (UV2075 plus, Jasco Co.) [13].
2.3. Assay for nattokinase activity
The nattokinase activity was measured by Japan Bio Science Laboratory Co. (1997) [14]. Briefly, 1.4 mL of 50mM KCl-H3BO3 buffer and 0.4 mL of 0.72 % of fibrinogen were incubated for 5 minutes at 37°C, and then 0.1 mL of thrombin (20 U/mL) was added to react for 10 minutes at 37°C. Subsequently, 0.1 mL of B. subtilis-fermented pigeon pea extract was added and incubated for 60 minutes at 37°C. The reaction was stopped with 2 mL of 0.2M trichloracetate, after centrifuging for 5 minutes (12,000 rpm). The absorption value was measured at 275 nm. The activity of nattokinase was calculated using the formula:
where AB = absorption value of blank; AS = absorption value of sample; D = sample dilute.
2.4. Assay for 2,2-diphenyl-1-picrylhydrazyl scavenging activity
The 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity was measured using the method described [15]. Briefly, B. subtilis-fermented pigeon pea extracts and DPPH methanolic solution were mixed and kept in the dark for 60 minutes. The absorbance of the reaction mixture at 517 nm was measured.
2.5. Assay for reducing power
The reducing power was measured using the approach of Duh and Yen [16]. B. subtilis-fermented pigeon pea extracts, phosphate buffer, and potassium hexacyanoferrate solution were mixed and heated at 50°C for 20 minutes and then trichloroacetic acid was added. Following centrifugation at 3000 g for 10 minutes, the supernatant was mixed with distilled water and ferric chloride, and the reaction was then maintained for 10 minutes. The absorbance at 700 nm was measured.
2.6. Assay for total antioxidant activity
The antioxidant capacity of B. subtilis-fermented pigeon pea extracts was measured, using the method of Miller and Rice-Evans [17]. Peroxidase, hydrogen peroxide, 2′-Azinobis [3-ethylbenzothiazoline-6-sulfonic acid]-diammonium salt (ABTS), and distilled water were mixed and stored in the dark for 1 hour at 25°C. A sample was subsequently added and the absorbance at 734 nm was determined.
2.7. Assay for total phenolic acid, flavonoids, and anthocyanin
The flavonoid contents were determined according to the modified method [18]. The sample was mixed with water, aluminium nitrate, and CH3COOH. The mixture was left in a dark room for 40 minutes and the absorbance was measured against blank at 415 nm using a spectrophotometer. Quercetin was used for the standard calibration curve and the flavonoid contents in the samples were calculated using the linear equation based on the calibration curve. Phenolic compounds were estimated using the method described by Singleton and Rossi [19] with modifications, and gallic acid as a standard phenolic compound. Briefly, the sample was mixed with Folin and Ciocalteu’s phenol reagent and sodium carbonate solution (7.5 %) was added and left for 90 minutes. The absorbance was measured at 760 nm. The results were expressed as mg of gallic acid equivalents/g of extract. Total anthocyanin content was measured using the pH differential method [20].
2.8. Animal experiment and experimental schedule
Male spontaneously hypertensive rats (SHRs, 5 weeks old; BioLASCO, Taiwan Co., Ltd., Yilan, Taiwan) were housed in individual plastic cages and subjected to a 12-hour light–dark cycle with 60% relative humidity at 25 ± 2°C. The animals were given free access to regular rodent chow diet and water for 1 week to adapt to the new environment. The experiments were carried out in a qualified animal breeding room in the animal center at our institute (protocol complied with guidelines described in the “Animal Protection Law”, amended on January 17th, 2001 Hua-Zong-(1)-Yi-Tzi-9000007530, Council of Agriculture, Executive Yuan, Taiwan, R.O.C.). The animal study was approved by the Institutional Animal Care and Use Committee of National Chiayi University. Animals were randomly divided into four groups (n = 6), including: (a) hypertensive SHRs; (b) SHRs + water extracts of pigeon pea [100 mg/kg body weight (bw)]; (c) SHRs + the water extracts of B. subtilis-fermented pigeon pea (100 mg/kg bw); and (d) SHRs + captopril (5 mg/kg bw), an ACE inhibitor. All samples were orally administered to SHRs for 8 weeks. Blood pressure of SHRs was measured once every 2-weeks continuously for 8 weeks.
2.9. Blood pressure measurements
After 1 week adaption in the new environment, at 0 hours, 4 hours, 8 hours, and 24 hours after a single oral administration of samples, individual rats were gently placed in a constant-temperature holder at 37 ± 1°C, and their systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured using tail cuff plethysmography with a photoelectric system (Visitech BP-2000, Napa Place, NC, USA) controlled with a personal computer. The mean value from at least five consecutive readings was used for the calculations. During the chronic administration, the SBP and DBP were measured before the weekly administration of samples.
2.10. Statistical analysis
Data were expressed as mean ± standard deviation. Statistical significance was determined using one-way analysis of variance with the general linear model procedure of SPSS Version 17.0 (SPSS Institute, Inc., Chicago, IL, USA), followed by one-way analysis of variance with Duncan’s test.
3. Results and discussion
3.1. Investigation of nattokinase activity
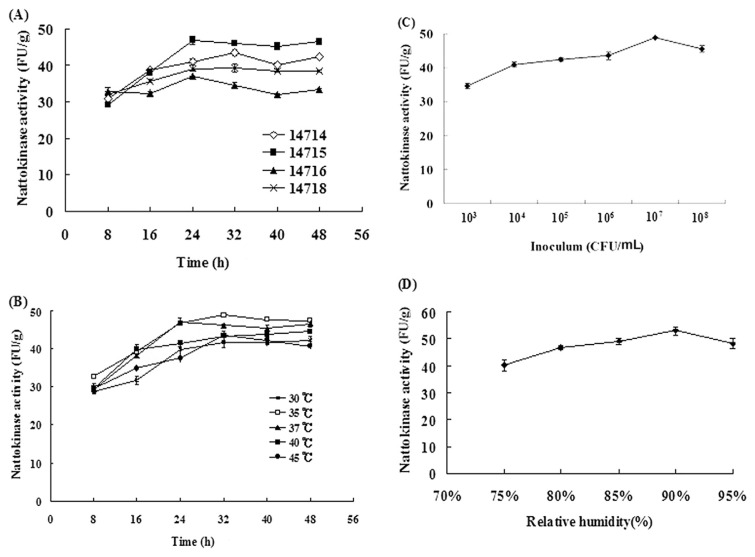
We have shown here that fermentation of pigeon pea by B. subtilis leads to increased production of nattokinase, which has been shown to have therapeutic effects in thrombosis. As shown in Fig. 1, generation of nattokinase was found during fermentation by various B. subtilis strains, including B. subtilis 14714, B. subtilis 14715, B. subtilis 14716, and B. subtilis 14718. The greatest nattokinase activity in pigeon pea was fermented by B. subtilis 14715 for 24 hours to 48 hours (Fig. 1A). For this B. subtilis strain, the optimal fermentation temperature was 35°C for 32 hours (Fig. 1B). An inoculum of 107 colony forming units/mL of B. subtilis 14715 could obtain the highest nattokinase activity (48.80 fibrolytic unit/g) after 32 hours fermentation (Fig. 1C). The optimal relative humidity of 90% yielded a nattokinase activity of 53.03 fibrolytic unit/g after 32 hours fermentation by B. subtilis 14715 (Fig. 1D).
Fig. 1.
Effects of (A) various strains; (B) various temperatures; (C) inoculum; and (D) relative humidity on nattokinase activity by Bacillus subtilis-fermented pigeon pea water extracts. Each value represents the mean ± standard deviation (n = 3). CFU = colony forming unit; FU = fibrolytic unit.
3.2. ACEI activity of water extracts from B. subtilis-fermented pigeon pea
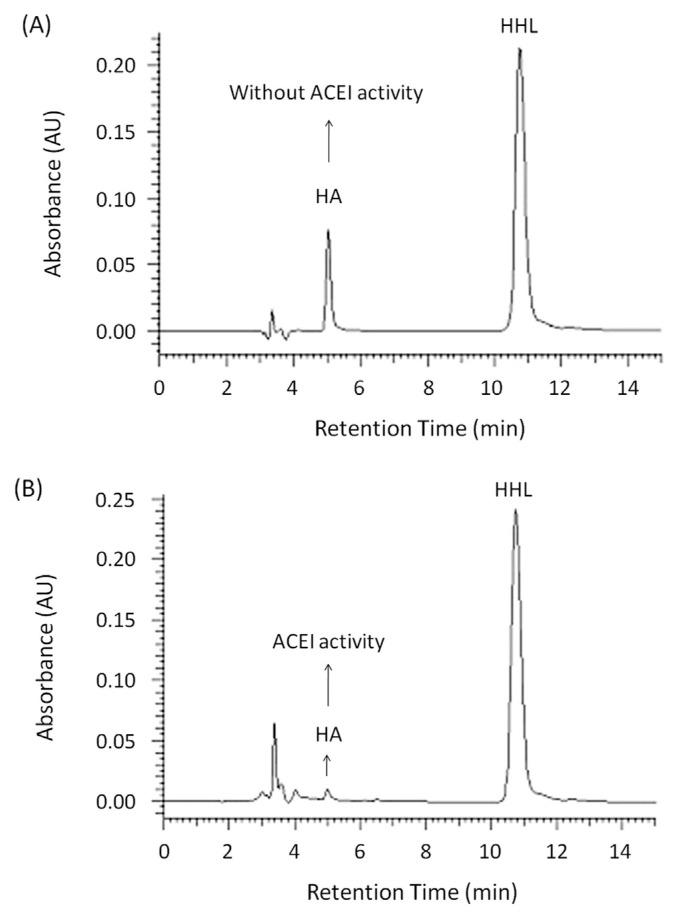
Angiotensin stimulates the release of aldosterone in the rennin-angiotensin system. It increases sodium and water reabsorption, leads to vasoconstriction, and increases blood pressure. Its inactive precursor, angiotensinogen, is converted by renin to inactive angiotensin I, and by ACE to angiotensin II. Milk is widely considered to be the substrate for lactic acid bacteria to produce ACE inhibitory peptides. A number of ACE inhibitory peptides have been shown to be effective in lowering the blood pressure of SHRs [21]. In this study, we found that B. subtilis 14715 fermentation of pigeon pea produced significant ACEI activity for 32 hours, with an EC50 of 1.47 mg/mL (Fig. 2). However, no such effect was found in nonfermented pigeon pea (data not shown).
Fig. 2.
Angiotensin-converting-enzyme inhibitory activity of water extracts from Bacillus subtilis-fermented pigeon pea. (A) Blank (without treatment with sample); and (B) treatment with water extracts of B. subtilis-fermented pigeon pea. Pigeon pea was fermented by B. subtilis 14715 for 32 hours. The EC50 of water extracts of B. subtilis-fermented pigeon pea on ACEI activity was 1.47 mg/mL. ACEI = angiotensin converting enzyme inhibition; HA = hippuric acid; HHL = hippuryl-His-Leu.
3.3. Antioxidant activity of water extracts from B. subtilis-fermented pigeon pea in vitro
Oxidative damage mediates the pathogenesis of atherosclerosis, a major contributor to CVDs worldwide. Lipid peroxides have an inhibitory effect on the activity of cholesterol esterase, an enzyme responsible for plaque formation during atherosclerosis. Previous studies have indicated that the modification of diet to include foods that contain antioxidants might prevent or ameliorate CVDs [22]. The role of oxidative stress in the pathogenesis of hypertension has been reported in several studies, and the main mechanism proposed is the reduced bioavailability of nitric oxide promoting endothelial dysfunction [23]. Elevated lipid peroxidation byproducts and decreased activity of antioxidant systems have been reported in hypertensive patients [23]. Several studies have indicated an increase in levels in hypertension and have implicated nicotinamide adenine dinucleotide phosphate-oxidase as a source of this excess [24].
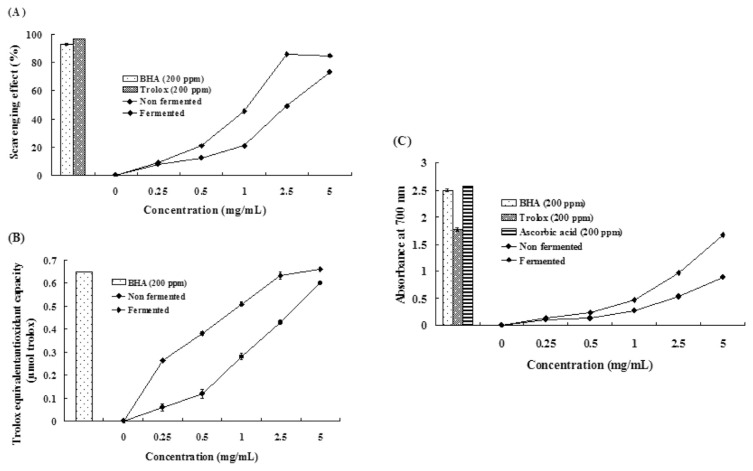
We next investigated the antioxidant activity of water extracts from B. subtilis 14715-fermented pigeon pea. The DPPH-scavenging activity, total antioxidant activity (trolox-equivalent antioxidant capacity), and reducing power were elevated in B. subtilis 14715-fermented pigeon pea compared with nonfermented pigeon pea in a dose-dependent manner (Fig. 3).
Fig. 3.
Effects of water extracts from Bacillus subtilis-fermented pigeon pea on: (A) 2,2-diphenyl-1-picrylhydrazyl scavenging activity; (B) total antioxidant activity (trolox equivalent antioxidant capacity); and (C) reducing powder. Each value represents the mean ± standard deviation (n = 3). Pigeon pea was fermented by B. subtilis 14715 for 32 hours. BHA = butylated hydroxyanisole.
3.4. Antioxidant of water extracts from B. subtilis-fermented pigeon pea
B. subtilis 14715 fermentation for 32 hours markedly increased the levels of total phenolic compounds (15.1 mg/g of water extracts) and flavonoids (5.39 mg/g of water extracts), whereas the anthocyanin level was decreased, compared with non-fermented pigeon pea (10.8 mg/g of phenolic compounds, 2.4 mg/g of pigeon pea water extracts) (Table 1). These results suggested that anthocyanin in pigeon pea was converted into flavonoids by B. subtilis 14715 fermentation.
Table 1.
Contents of total phenolics, flavonoids, and anthocyanin of nonfermented Bascillus subtilis-pigeon pea and fermented B. subtilis-pigeon pea.
| Sample | Contentsa | ||
|---|---|---|---|
|
| |||
| Total phenolic compounds (mg/g) | Flavonoids (mg/g) | Anthocyanin (mg/g) | |
| Nonfermented | 10.83 ± 0.63 | 2.45 ± 1.12 | 1.05 ± 0.04 |
| Fermentedb | 15.15 ± 0.50 | 5.39 ± 0.76 | 0.85 ± 0.11 |
Values are the mean ± standard deviation (n = 3). Data bearing different superscript letters in the same column are significantly different (p < 0.05).
Pigeon pea was fermented by B. subtilis 14715 for 32 hours.
Not only did we find increased nattokinase activity in water extracts from B. subtilis 14715-fermented pigeon pea, but also increased antioxidant capacity and ACEI activity. These findings suggest that extracts of B. subtilis-fermented pigeon pea may also have antihypertension potential, in addition to its antiatherosclerosis potential, and these results are similar to those found for extracts of natto [25].
3.5. Antihypertensive activity of water extracts from B. subtilis-fermented pigeon pea
Growing evidence suggests that increased oxidative stress is involved in the pathogenesis of CVD in metabolic syndrome. Oxidative stress, which may occur as a consequence of the imbalance between free radical production and the capacity of cellular antioxidant systems, induces cell damage and the deregulated production of adipocytokines, which contributes to obesity-associated insulin resistance and hypertension [26].
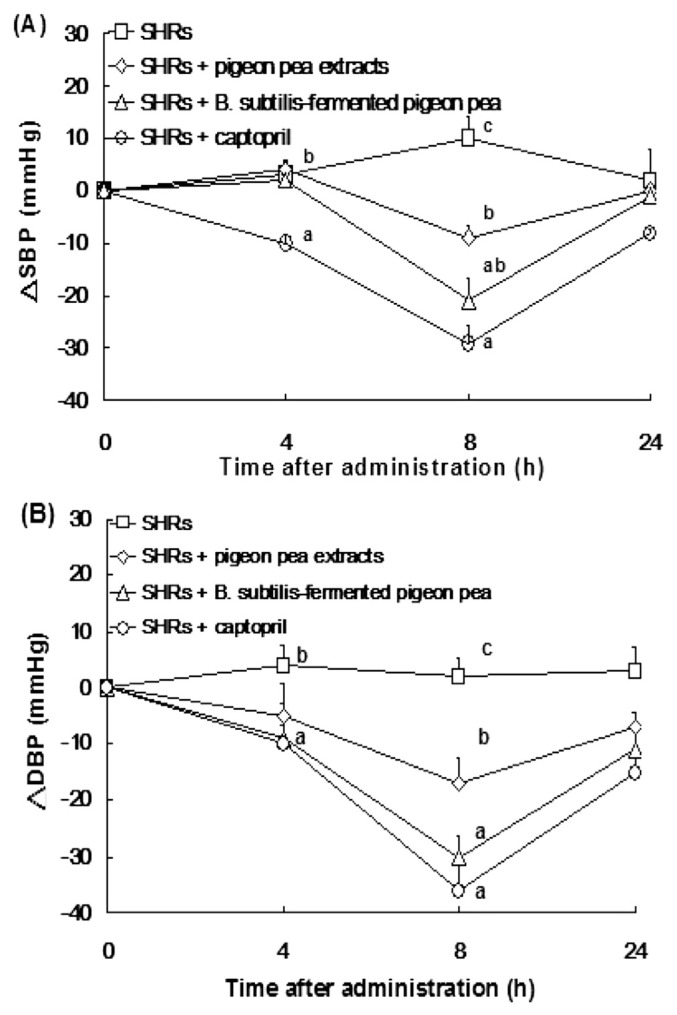
Polyphenol-rich food has been reported to show anti-hypertension effect by ACEI activity in vitro [27]. Antioxidants also improve endothelium-dependent vasodilatation of mesenteric arteries in SHRs [26]. Currently, we found that fermentation of pigeon pea with B. subtilis 14715 increased nattokinase activity, as well as antioxidant levels (phenolics and flavonoids) and ACEI activity, compared with those in nonfermented pigeon pea extracts. In our in vivo model, the data showed that the water extracts of B. subtilis-fermented pigeon pea (100 mg/kg bw) markedly attenuated SBP and DBP after administration by 21 mmHg and 30 mmHg for 8 hours, respectively; this effect was greater than the administration of water extracts of pigeon pea (100 mg/kg bw) in SBP and DBP by 9 mmHg and 17 mmHg, respectively. SBP and DBP were both decreased by 29 mmHg and 36 mmHg in captopril-administered SHRs (Fig. 4).
Fig. 4.
Effect of a single oral administration of pigeon pea on: (A) systolic blood pressure; and (B) diastolic blood pressure in spontaneous hypertensive rats (SHRs). Animals were randomly divided into four groups (n = 6; significant difference was shown by different letters), including: hypertensive SHRs; SHRs + water extracts of pigeon pea [100 mg/kg body weight (bw)]; SHRs + the water extracts of Bacillus subtilis-fermented pigeon pea (100 mg/kg bw); and SHRs + captopril (5 mg/kg bw). Each value represents the mean ± standard error of the mean. Both systolic blood pressure and diastolic blood pressure were measured after the start (at 6 weeks of age) of chronic feeding with the water extracts of B. subtilis-fermented pigeon pea or the water extracts of pigeon pea. DBP = diastolic blood pressure; SBP = systolic blood pressure.
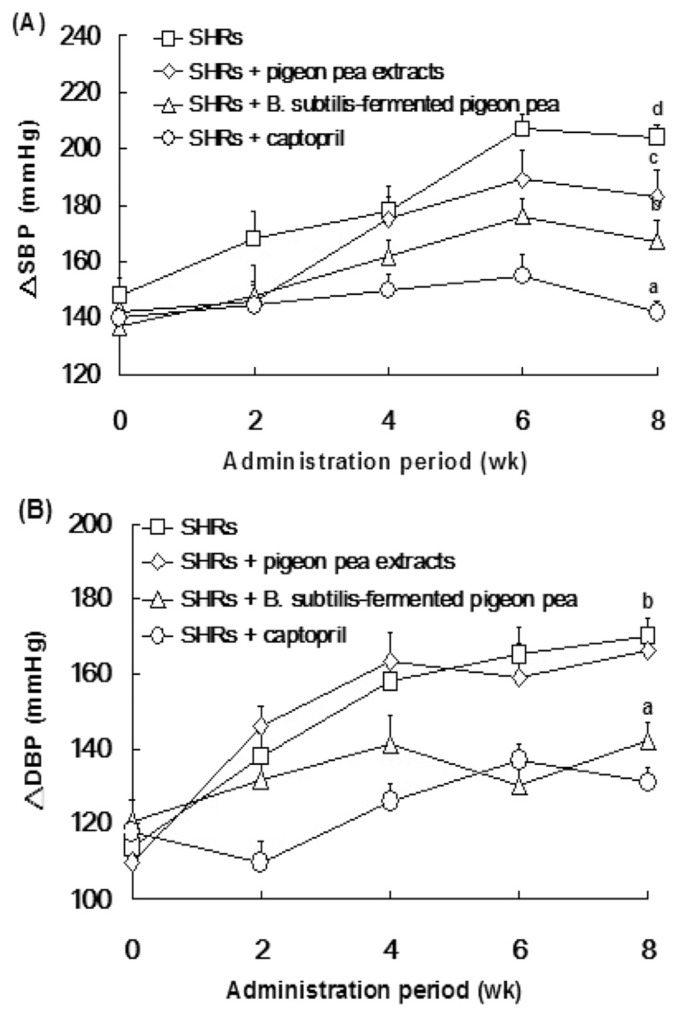
In addition, Fig. 5 shows changes in the average resting SBP and DSP throughout the administration period (0–8 weeks). SBP and DBP were 167 mmHg and 142 mmHg in the water extracts of B. subtilis-fermented pigeon pea-treated SHRs at Week 8, respectively, however, SBP and DBP were 183 mmHg and 166 mmHg in the water extracts of pigeon pea, respectively. These findings indicated that ACEI activity, nattokinase, and antioxidative activities of pigeon pea fermented by B. subtilis were able to decrease SBP and DBP, thereby exerting antihypertension potential, suggesting B. subtilis-fermented pigeon pea has benefits for cardiovascular health.
Fig. 5.
Antihypertensive effects of the water extracts from Bacillus subtilis-fermented pigeon pea or the water extracts from pigeon pea. Animals were randomly divided into four groups (n = 6), including: (a) hypertensive spontaneous hypertensive rats (SHRs); (b) SHRs +water extracts of pigeon pea [100 mg/kg body weight (bw)]; (c) SHRs + the water extracts of B. subtilis-fermented pigeon pea (100 mg/kg bw); and (d) SHRs +captopril (5 mg/kg bw). Each value represents the mean ± standard error of the mean. All samples were orally administered to SHRs for 8 weeks. Blood pressure of SHRs was measured once every 2-weeks continuously for 8 weeks. DBP = diastolic blood pressure; SBP = systolic blood pressure.
Phytochemicals such as resveratrol (30 mg/kg bw) has been reported to attenuate oxidative stress-induced hypertension [28]. ACEI peptides of milk or lactic acid bacteria-fermented products have been reported to attenuate hypertension in recent studies [29,30]. Monascus-fermented products have also been shown to improve hypertension in SHRs, as well as alter the production of ACEI peptides [31].
Inflammation might contribute to the acceleration of vascular damage and hypertension and activate the reninangiotensin system [32]. Pigeon pea has anti-inflammatory effects that may contribute to the repression of vascular inflammation and proinflammatory molecules in the endothelial wall, such as vascular cell adhesion molecule-1, inter-cellular adhesion molecule, and E-selectin, and these molecules may lead to hypertension [32]. In a recent study, we found that pigeon pea was able to inhibit inflammation [12]. Currently, our results also suggest that pigeon pea may prevent hypertension mediated by elevating ACEI activity, nattokinase, and antioxidation in SHRs.
4. Conclusion
The beneficial effects of extracts from B. subtilis-fermented pigeon pea on ACEI activity, antioxidant levels, and nattokinase generation were examined in this study. Extracts from B. subtilis-fermented pigeon pea significantly elevated antioxidant levels, and raised nattokinase and ACEI activities. These results suggest that B. subtilis-fermented pigeon pea may be beneficial to cardiovascular health, and can potentially be developed as a new dietary supplement or marketed as a functional food with disease-prevention properties.
Acknowledgments
This research work and subsidiary spending were supported by the Council of Agriculture, R.O.C., under Grant DOH098-TD-F-113-098018.
Funding Statement
This research work and subsidiary spending were supported by the Council of Agriculture, R.O.C., under Grant DOH098-TD-F-113-098018.
Footnotes
Conflicts of interest
All contributing authors declare no conflicts of interest.
REFERENCES
- 1. Aukrust P, Halvorsen B, Yndestad A, Ueland T, Oie F, Otterdal K. Chemokines and cardiovascular risk. Arterioscler Thromb Vascu Biol. 2008;28:1909–19. doi: 10.1161/ATVBAHA.107.161240. [DOI] [PubMed] [Google Scholar]
- 2. Anderson JW, Smith BM, Washnock CS. Cardiovascular and renal benefits of dry bean and soybean intake. Am J Clin Nutr. 1999;70:464–74. doi: 10.1093/ajcn/70.3.464s. [DOI] [PubMed] [Google Scholar]
- 3. Suzuki Y, Kondo K, Ichise H, Tsukamoto Y, Urano T, Umemura K. Dietary supplementation with fermented soybeans suppresses intimal thickening. Nutrition. 2003;19:261–4. doi: 10.1016/s0899-9007(02)00853-5. [DOI] [PubMed] [Google Scholar]
- 4. Rivera-Espinoza Y, Gallardo-Navarro Y. Non-dairy probiotic products. Food Microbiol. 2010;27:1–11. doi: 10.1016/j.fm.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 5. Tai MW, Sweet BV. Nattokinase for prevention of thrombosis. Am J Health-Syst Pharma. 2006;63:1121–3. doi: 10.2146/ajhp050509. [DOI] [PubMed] [Google Scholar]
- 6. Juan MY, Wu CH, Chou CC. Fermentation with Bacillus spp. as a bioprocess to enhance anthocyanin content, the angiotensin converting enzyme inhibitory effect, and the reducing activity of black soybeans. Food Microbiol. 2010;27:918–23. doi: 10.1016/j.fm.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 7. Hsia CH, Shen MC, Lin JS, Wen YK, Hwang KL, Cham TM. Nattokinase decrease plasma levels of fibrinogen, factor VII, and factor VIII in human subjects. Nutr Res. 2009;29:190–6. doi: 10.1016/j.nutres.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 8. Miettinen TA, Tilvis RS, Kesaniemi YA. Serum plant sterols and cholesterol precursors reflect cholesterol absorption and synthesis in volunteers of a randomly selected male population. Am J Epidemiol. 1990;131:20–31. doi: 10.1093/oxfordjournals.aje.a115479. [DOI] [PubMed] [Google Scholar]
- 9. Ambekar S, Patil SC, Giri AP, Kachole MS. Proteinaceous inhibitors of trypsin and amylases in developing and germinating seeds of red gram (Cajanus cajan L Millsp) J Sci Food Agric. 1996;72:57–62. [Google Scholar]
- 10. Grover JK, Yadav S, Vats JJ. Medicinal plants of India with anti-diabetic potential. J Ethnopharmacol. 2002;81:81–100. doi: 10.1016/s0378-8741(02)00059-4. [DOI] [PubMed] [Google Scholar]
- 11. Ghosh A, Sarkar K, Sil PC. Protective effect of a 43kD protein from the leaves of the herb, Cajanus indicus L on chloroform induced hepatic-disorder. J Biochem Mol Biol. 2006;39:197–207. doi: 10.5483/bmbrep.2006.39.2.197. [DOI] [PubMed] [Google Scholar]
- 12. Lai YS, Hsu WH, Huang JJ, Wu SC. Antioxidant and anti-inflammatory effects of pigeon pea (Cajanus cajan L.) extracts on hydrogen peroxide- and lipopolysaccharide-treated RAW264.7 macrophages. Food Funct. 2012;3:1294–301. doi: 10.1039/c2fo30120b. [DOI] [PubMed] [Google Scholar]
- 13. Wu CL, Lee CL, Pan TM. Red mold dioscorea has a greater antihypertensive effect than traditional red mold rice in spontaneously hypertensive rats. J Agric Food Chem. 2009;57:5035–41. doi: 10.1021/jf900349v. [DOI] [PubMed] [Google Scholar]
- 14.Japan Bio Laboratory Co., Ltd. Assay method of nattokinase. 1997. http://jbsl-net.com/english/products/index.html .
- 15. Shimada K, Fujikawa K, Yahara K, Nakamura T. Antioxidative properties of xanthan on the autooxidation of soybean oil in cyclodextrin. J Agric Food Chem. 1992;40:945–8. [Google Scholar]
- 16. Duh PD, Yen GC. Antioxidative activity of three herbal water extracts. Food Chem. 1997;60:639–45. [Google Scholar]
- 17. Miller NJ, Rice-Evans CA. The relative contributions of ascorbic acid and phenolic antioxidants to the total antioxidant activity of orange and apple fruit juices and black currant drink. Food Chem. 1997;60:331–7. [Google Scholar]
- 18. Jia Z, Tang M, Wu J. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999;64:555–9. [Google Scholar]
- 19. Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid regent. Am J Enol Viticult. 1965;16:144–58. [Google Scholar]
- 20. Fuleki T, Francis FJ. Quantitative methods for anthocyanins. Extraction and determination of total anthocyanin in cranberries. J Food Sci. 1968;33:72–7. [Google Scholar]
- 21. Masuda O, Nakamura Y, Takano T. Antihypertensive peptides are present in aorta after oral administration of sour milk containing these peptides to spontaneously hypertensive rats. J Nutr. 1996;126:3063–8. doi: 10.1093/jn/126.12.3063. [DOI] [PubMed] [Google Scholar]
- 22. Nagarajan S. Mechanisms of antiatherosclerotic functions of soy based diets. J Nutr Biochem. 2010;21:255–60. doi: 10.1016/j.jnutbio.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 23. Roberts CK, Sindhu KK. Oxidative stress and metabolic syndrome. Life Sci. 2009;84:705–12. doi: 10.1016/j.lfs.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 24. Touyz RM, Schiffrin EL. Increased generation of superoxide by angiotensin II in smooth muscle cells from resistance arteries of hypertensive patients: role of phospholipase D-dependent NAD(P)H oxidase-sensitive pathways. J Hyperten. 2001;19:1245–54. doi: 10.1097/00004872-200107000-00009. [DOI] [PubMed] [Google Scholar]
- 25. Chang CH, Chen KT, Lee TH, Wang CH, Kuo YW, Chiu YH, Hsieh CL, Wu CJ, Chang YL. Effects of natto extract on endothelial injury in a rat model. Acta Medica Okayama. 2010;64:399–406. doi: 10.18926/AMO/41326. [DOI] [PubMed] [Google Scholar]
- 26. Otani H. Oxidative stress as pathogenesis of cardiovascular risk associated with metabolic syndrome. Antioxid Redox Signal. 2011;15:1911–26. doi: 10.1089/ars.2010.3739. [DOI] [PubMed] [Google Scholar]
- 27. Oboh G, Ademiluyi AO, Akinyemi AJ, Henle T, Saliu JA, Schwarzenbolz U. Inhibitory effect of polyphenol-rich exptreacts of jute leaf (Corchorus olitorius) on key enzyme linked to type 2 diabetes (alpha-amylase and alpha-glucosidase) and hypertension (angiotensin I converting) in vitro. J Funct Foods. 2012;4:450–8. [Google Scholar]
- 28. Franco JG, Lisboa PC, Lima NS, Amaral TAS, Peixoto-Silva N, Resende AC, Oliveira E, Passos MCF, Moura EG. Resveratrol attenuates oxidative stress and prevents steatosis and hypertension in obese rats programmed by early weaning. J Nutr Biochem. 2013;24:960–6. doi: 10.1016/j.jnutbio.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 29. Liu CF, Tung YT, Wu CL, Lee BH, Hsu WH, Pan TM. Antihypertensive effects of Lactobacillus-fermented milk orally administered to spontaneously hypertensive rats. J Agric Food Chem. 2011;59:4537–43. doi: 10.1021/jf104985v. [DOI] [PubMed] [Google Scholar]
- 30. Patten GS, Abeywardena MY, Head RJ, Bennett LE. Processed dietary plants demonstrate broad capacity for angiotensin converting enzyme and angiotensin II receptor binding inhibition in vitro. J Funct Foods. 2012;4:851–63. [Google Scholar]
- 31. Brown NJ, Vaughan DE. Angiotensin-converting enzyme inhibitors. Circulation. 1998;97:1411–20. doi: 10.1161/01.cir.97.14.1411. [DOI] [PubMed] [Google Scholar]
- 32. Boumaza S, Arribas SM, Osborne-Pellegrin M, McGrath JC, Laurent S, Lacolley P, Challande P. Fenestrations of the carotid internal elastic lamina and structural adaptation in stroke-prone spontaneously hypertensive rats. Hypertension. 2001;37:1101–7. doi: 10.1161/01.hyp.37.4.1101. [DOI] [PubMed] [Google Scholar]