Abstract
Objective
To describe effectiveness of mRNA vaccines by comparing 2-dose (2D) and 3-dose (3D) healthcare worker (HCW) recipients in the setting of Omicron variant dominance. Performance of 2D and 3D vaccine series against SARS-CoV-2 variants and the clinical outcomes of HCWs may inform return-to-work guidance.
Methods
In a retrospective study from December 15, 2020 to January 15, 2022, SARS-CoV-2 infections among HCWs at a large tertiary cancer centre in New York City were examined to estimate infection rates (aggregated positive tests / person-days) and 95% CIs over the Omicron period in 3D and 2D mRNA vaccinated HCWs and were compared using rate ratios. We described the clinical features of post-vaccine infections and impact of prior (pre-Omicron) COVID infection on vaccine effectiveness.
Results
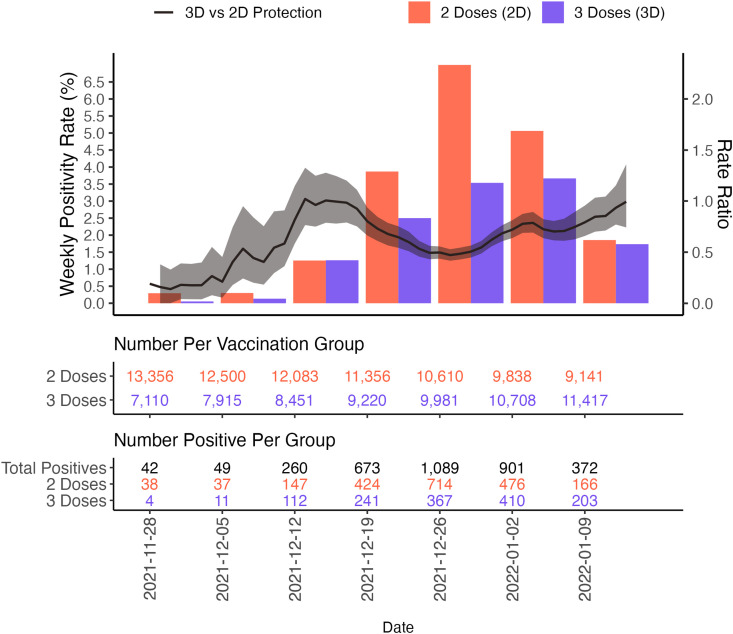
Among the 20857 HCWs in our cohort, 20,660 completed the 2D series with an mRNA vaccine during our study period and 12461 had received a third dose by January 15, 2022. The infection rate ratio for 3D versus 2D vaccinated HCWs was 0.667 (95% CI 0.623, 0.713) for an estimated 3D vaccine effectiveness of 33.3% compared to two doses only during the Omicron dominant period from December 15, 2021 to January 15, 2022. Breakthrough Omicron infections after 3D + 14 days occurred in 1,315 HCWs. Omicron infections were mild, with 16% of 3D and 11% 2D HCWs being asymptomatic.
Discussion
Study demonstrates improved vaccine-derived protection against COVID-19 infection in 3D versus 2D mRNA vaccinees during the Omicron surge. The advantage of 3D vaccination was maintained irrespective of prior COVID-19 infection status.
Keywords: Booster breakthrough infections, SARS-CoV-2, Vaccine effectiveness
Introduction
The Omicron variant (B.1.1.529 and associated lineages B.1, B1.1, B.2, and B.3) can evade vaccine and natural immunity due in part to several mutations in the spike protein region [[1], [2], [3], [4], [5]]. First identified in November 2021 in Botswana and South Africa, Omicron was designated a variant of concern by the World Health Organization within a month after its emergence [6]. The United States identified its first Omicron case on December 1, 2021, and the Northeast region including New York State witnessed the earliest and steepest rise in case numbers, including among vaccinated individuals.
Prior to Omicron we previously reported on the effectiveness of COVID-19 mRNA vaccines BNT162b2 (BioNTech and Pfizer) and mRNA-1273 (Moderna) in a cohort of NYC HCWs during the Alpha and Delta surges. Our results demonstrated high clinical effectiveness of the mRNA vaccines, with minimal waning of protection against mild–moderate Delta infection [7]. The observed higher transmissibility of Omicron variant compared to Delta among vaccinated individuals requires a re-evaluation of the effectiveness of 2-dose (2D) and 3-dose (3D) mRNA vaccines among U.S. HCWs. This study compared SARS-Cov-2 infection rates among 2D and 3D mRNA vaccinated NYC healthcare workers during the Omicron surge in December 2021 to January 2022. We also described the clinical characteristics of cases and assessed the effect of prior COVID infection on vaccine effectiveness.
Methods
Memorial Sloan Kettering Cancer Center (MSKCC) is a 514-bed tertiary cancer centre in New York City that employs >21 000 individuals. A COVID vaccine mandate (for 2D primary series) went into effect for New York State HCWs on September 21, 2021. The CDC recommended 3D for U.S. HCWs on September 24, 2021, and the MSKCC made them available on September 27th, 2021 (Fig. S1).
HCW testing policy
In response to the Omicron surge, HCW testing was prioritized for symptomatic employees. Testing in the community was encouraged for asymptomatic HCWs with nonworkplace-related exposures. Workplace exposures were limited due to universal masking. All positive results, regardless of testing method, required reporting to MSKCC Employee Health and Wellness Service through an automated survey. Clinical characteristics of HCW infections were obtained from a dedicated Employee Health and Wellness Service database deployed for symptom self-reporting through an electronic questionnaire during the Omicron surge, whereas they were previously collected during clinician interviews for contact tracing and return-to-work clearance.
Laboratory methods
SARS-CoV-2 RNA test
For HCWs tested at MSKCC, the COVID-19 diagnosis was made through detection of viral RNA in nasopharyngeal swabs or saliva samples using two real-time reverse transcriptase PCR tests: the Taq Path COVID-19 Combo Kit (Thermo Fisher Scientific, Waltham, MA) or the Cobas SARS-CoV-2 test (Roche Molecular Diagnostics, Indianapolis, IN). The performance of these real-time reverse transcriptase PCR on saliva samples was previously described [8,9]. The samples were reported as positive per manufacturers' instructions.
Statistical analysis
To evaluate the impact of three mRNA doses on protection against Omicron variant, we compared the estimates of test positivity rates among 3D + ≥14 days and 2D + ≥14 days vaccinated HCWs during the NYC Omicron surge from December 15, 2021 to January 15, 2022 (Fig. S3). To estimate the incidence rates in these groups, a rolling risk set was defined each day consisting of all currently employed HCWs on that day. If the HCWs ended their employment at MSKCC, they were removed from the rolling cohort due to incomplete access to vaccination and testing records. Newly hired employees entered the cohort at date of hire, as vaccination records were known but testing information was not usually available prior to their employment. Temporary contingent employees were excluded in the rate estimates due to incomplete records. Other HCW exclusion criteria are outlined in Fig. S2. Using this rolling cohort, we estimated the rate ratio of total number of positive tests in each vaccination group over the number of person-days in each vaccination category. The 95% CIs were estimated for rate ratios using 1000 bootstrap iterations and the percentile method to approximate confidence bounds. “Effectiveness” (percent relative effect) was calculated as 1 minus the rate ratio.
To adjust for changing background community infection rates and to visualize potential variations in vaccine effectiveness over the course of Omicron period, daily and weekly (daily estimates X 7) incidence rates were calculated by dividing the total number of positive tests each day by the number of HCWs in each vaccination category each day. The rates were smoothed using a 7-day moving window.
For analysis of the potential effect of prior COVID-19 positives on vaccine effectiveness, a subset of employees who were employed since November 1, 2020 were used, including individuals with prior infection with any variant. Among this subset, the vaccination categories were further broken down into the following groups: 1) 3D + ≥14 days without prior infection, 2) 3D + ≥14 days with prior infection, 3) 2D + ≥14 days without prior infection, and 4) 2D + ≥14 days with prior infection. The rate ratios were calculated between these groups as described above.
For all analyses, any HCWs with positives tests within 90 days of each other were presumed to be part of the same infection, except for two HCWs who had infections on either side of the transition date from Delta to Omicron dominance in NYC and who reported distinct clinical syndromes. The 3D vaccine breakthrough (BT) infections were defined as the detection of SARS-COV-2 RNA in a respiratory or saliva specimen or a positive antigen test in a nasal swab (performed on or after December 1, 2021) collected from an HCW ≥14 days after receipt of 3D mRNA vaccine and >28 days from completion of primary two-dose vaccine series. All analyses were conducted in R version 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria).
The MSKCC Institutional Review Board granted a HIPAA approval to conduct the study.
Results
Rate ratio calculations
Of the 21,557 HCWs employed at MSKCC as of December 15, 2021 and eligible for inclusion, 20 857 were included in the evaluable cohort for incidence rate ratio calculations. As of the study end date (January 15, 2022) 20,864 HCWs were employed at the study institution. Of these, 20 700 (99.2%) had recorded at least one dose of a COVID vaccine, 20 660 (99.0%) had recorded two doses, and 12 461 (59.7%) had a record of three doses. One percent of HCWs in the cohort had a medical exemption from the vaccine mandate or were newly hired and completing their initial vaccine series. Among 2D recipients employed at study end, the median time from administration of second dose to study end was 11.2 months days (IQR 8.9–11.6 months). Among 3D recipients, median time from administration of third dose to study end was 67 days (IQR 38–87 days). The HCWs who received a third dose were more likely to have at least one COVID test on record compared to those who did not (79% vs. 70%, p < 0.001) (Fig. S4).
Infections after 2D and 3D
Laboratory-confirmed SARS CoV-2 infections occurred in 3203 vaccinated individuals during the Omicron dominant period (December 15, 2021 to January 15, 2022), including 1315 infections in HCWs who had received 3D + ≥14 days and 1888 who received 2D + ≥14 days. In contrast, there were 509 infections in vaccinated individuals during the Delta dominant period (July 1, 2021 to December 14, 2021), 36 of which occurred in HCWs with 3D + ≥14 days and 473 of which occurred in HCW 2D + ≥14 days.
Overall, there were 1351 3D BT cases across both the Delta and Omicron dominant periods. Among these 3D BT cases, the median time between booster shot and positive SARS-CoV-2 test was 62 days (IQR 44–78 days).
Fig. 1 depicts the weekly rate ratios for infections in 3D + ≥14 days versus 2D + ≥14 days recipients across the period of booster availability at the study institution. The overall rate ratio comparing 3D protection to 2D protection was 0.667 (95% CI, 0.623, 0.713).
Fig. 1.
Weekly rate ratios for infections in 3D vs 2D HCW vaccine recipients.
The comparative vaccine effectiveness of 3D versus 2D for lab-confirmed SARS CoV-2 infection for the Omicron dominant period (after December 15, 2021) was 33.3% (95% CI, 28.7%, 37.7%).
Impact of prior COVID infection on BT infections
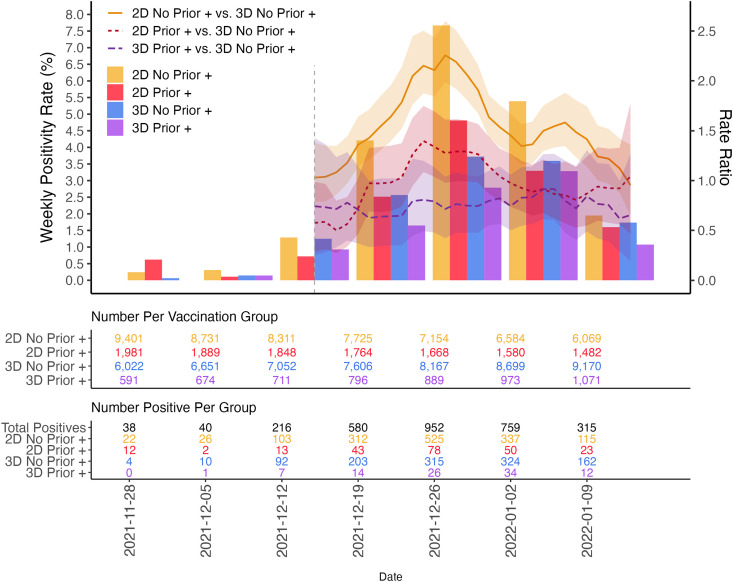
Of all 3D BT infections during Delta and Omicron periods, 7.2% (98/1351) had a record of a prior COVID infection. Of 1315 3D breakthrough infections during the Omicron period, 94 (7.1%) had a previous COVID infection. In contrast, for 1888 2D BTs during Omicron period, 209 (11.1%) had a prior COVID infection. Of the 21 557 HCWs employed at the start of Omicron, 18 152 had been employees since at least November 1, 2020 and therefore had adequate COVID testing records to examine effects of prior positives on rate ratios. In this subset, 7.9% (93/1172) of 2D BTs had a recorded prior positive and 13.1% (205/1570) of 2D BTs had a prior positive.
The rate ratios comparing incidence rates among those with a prior documented positive test of any variant with those with no prior infection were calculated between each vaccine group (Fig. 2 ). Within the 2D and 3D groups, the rate ratios calculated from December 15, 2021 to January 15, 2022 comparing those who had an infection prior to their Omicron infection versus those who did not were <1, indicating an additional protective effect of a prior infection (2D Prior + vs. 2D no prior +: 0.635 (0.545, 0.733); 3D prior + vs. 3D no prior +: 0.768 (0.622, 0.926); Table S1). For patients with prior infection, protection against Omicron was better with three doses than two doses (3D prior + infection vs. 2D prior + infection 0.757 (0.587, 0.935), highlighting the added benefit of an additional vaccine dose to the protection derived from prior infection.
Fig. 2.
Rate ratios comparing positivity rates by vaccine status and prior documented infection.
Clinical and demographic characteristics of HCWs with 3D infections
Eighty percent (n = 1061) of HCWs with 3D breakthrough infections with Omicron had completed the symptom survey. The respondent median age was 34 years (range 20–78 years). Sore throat (63%) and cough (54%) were the predominant symptoms. Omicron infections were mild, with 16% of 3D and 11% 2DHCWs being asymptomatic. No hospitalizations were noted in 3D recipients compared to one reported among 2D recipients (Table S2). No COVID-related deaths were reported in either group during the Omicron surge.
Discussion
Our study from an established cohort of over 20 000 NYC HCW showed a benefit of 3D versus 2D of mRNA vaccine against infection caused by the Omicron variant. The comparative vaccine efficacy (VE) of 3D versus 2D against illness was 33.3%. No hospitalizations or severe infections were reported among 3D vaccinees. Further, our data suggested that HCWs with prior COVID-19 booster doses may provide additional protection against Omicron infection.
The sudden unexpected emergence of the Omicron variant challenged U.S. healthcare systems because of the sheer scale of spread in patients and HCWs. The higher transmissibility of the variant fuelled this among vaccinated and previously infected individuals due to its immune evasion properties and concerns about waning immunity of the primary 2D vaccine series [2,5,10]. The life-saving impact of booster vaccination against the dynamic COVID-19 landscape cannot be underestimated [11]. Understanding the vaccine effectiveness and subsequent impact of Omicron in a highly vaccinated healthcare workforce may elucidate how healthcare systems can pre-emptively manage future surges, devise effective vaccination policies, and combat vaccine hesitancy.
Third doses for HCW were approved in the United States with some delay compared to other countries. A systematic assessment of booster vaccine effectiveness among U.S. HCW is limited, especially against Omicron, despite growing evidence of the benefits of three mRNA vaccine doses in other populations. Data from Israel, where waning protection against illness and severe disease prompted booster administration in July 2021, demonstrated restored protection across all age groups [12,13]. Further, preliminary studies have supported higher vaccine effectiveness of booster doses for HCWs among nearly 2000 HCWs at a single medical centre in Israel (3D vs. 2D recipients infection rates at 39 days after booster, 12.8 vs. 116 per 100 000 person-days) [14,15].
Consistent with reports on immune evasiveness of Omicron, recent data from the United Kingdom's Public Health England (PHE) show that the vaccine effectiveness against symptomatic Omicron infection was much lower than against the Delta variant in boosted individuals [16]. Protection against hospitalization was sustained: 75% to 90% VE for Pfizer 3D recipients and >90% with Moderna with an 8- to 9-week follow-up. Similarly, the reports from the United States showed slightly lower vaccine effectiveness of the booster dose against emergency department visits and hospitalization for Omicron compared to Delta [15]. Our other important finding demonstrated slightly improved clinical protection against Omicron with the third dose among HCW with prior COVID-19 infection compared to those with two doses only. Although studies should confirm these findings in other populations, the observations were consistent with emerging evidence on the benefit of vaccination even in those with pre-Omicron infection. For example, in a review of registry data from California and New York through November 2021, the case rates for infection were substantially lower among vaccinated persons with a prior COVID-19 diagnosis [17]. Previous COVID-19 infection is one of the reasons for booster hesitancy. Although vaccination safety in those with prior infection is well established, we provided early evidence that suggested better clinical protection against Omicron in 3D vaccinated persons with prior infection.
Evaluating vaccine effectiveness during a novel variant surge is subject to several limitations. Availability of PCR testing was adjusted to prioritize symptomatic testing, which may have led to an underestimate of asymptomatic Omicron infections across both 2D and 3D HCWs, despite accessibility of home testing. Additionally, symptom assessment was conducted only at disease onset and via optional self-reported survey, which may have led to increased symptom reporting in those with health-seeking behaviour. Boosted individuals sought testing and tested more often, reflecting a potential bias toward healthcare-seeking behaviour in the 3D group, and which may have resulted in under-surveillance of infections in the 2D group. Although 3D HCW had more tests overall, 2D HCW testing rates disproportionately increased during the last 2 weeks of 2021 and first week of 2022 corresponding to many holidays (Fig. S4). This increase in testing surveillance occurred concurrently with an increase in incidence rates in the 2D group and a decrease in rate ratios when comparing 2D to 3D HCWs in those same weeks and thus more accurately reflect VE of 3D in our cohort.
Additionally, the estimates of positive test incidence rates may be confounded by age or job type. A sensitivity analysis estimating stratum-specific rate ratios showed comparable estimates of effectiveness among these strata and compared with the overall estimates presented in the paper (<5% difference, Table S3).
We also interrogated the possibility of inflated estimates of 2D protection due to the inclusion of 3D reipients <14 days out from their third dose, removing these individuals that showed rate ratios comparable to the ones presented in the main study (0.631 95% CI: 0.591, 0.671).
Delta and Omicron cocirculated in NYC very briefly, which may have led to inconsequential variant miscategorization in early December.
In summary, our study confirmed that HCWs vaccinated with three doses have better protection against Omicron infection than those immunized with only two doses. Further, the vaccination enhances clinical protection in those with prior COVID-19 infection. Therefore, U.S. jurisdictions should adopt HCW booster mandate programs more broadly to optimize protection against emerging variants.
Transparency declaration
SF has received payment or honouraria from Amgen, GSK, Daiichi, Astra Zeneca, G1, Coherus, and Regeneron outside the scope of this work. NEB reports grants from GenMark Dx and personal fees from Roche Diagnostics, outside the submitted work.
Funding was supported by the Memorial Sloan Kettering Cancer Center core grant (P30 CA008748), the Jack and Dorothy Byrne Family Fund (NEB, MK), Department of Medicine, Memorial Sloan Kettering Cancer Center, and Weill Cornell Medicine. All other authors declare no conflicts of interests.
Author contributions
MK conceptualized this project. EVR, KW, AL, and CP performed data curation. KW and VS completed formal analysis. KJ, TM, and NEB conducted sequencing as part of investigation to assign variant lineages. EVR wrote the original draft and all authors provided critical review and commentary on all drafts. EVR and KW contributed equally to this article.
Editor: J. Rodriguez-Baño
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2022.07.017.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Moline H.L., Whitaker M., Deng L., Rhodes J.C., Milucky J., Pham H., et al. Effectiveness of COVID-19 vaccines in preventing hospitalization among adults aged >/=65 years–COVID-NET, 13 states. MMWR Morb Mortal Wkly Rep. 2021;70:1088–1093. doi: 10.15585/mmwr.mm7032e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ferguson N., Ghani A., Cori A., Hogan A., Hinslet W., Volz E. Imperial College London; 2021. Report 49: growth, population distribution and immune escape of the Omicron in England. [Google Scholar]
- 3.Liu L., Iketani S., Guo Y., Chan J.F., Wang M., Liu L., et al. Striking antibody evasion manifested by the Omicron variant of SARS-CoV-2. Nature. 2022;602:676–681. doi: 10.1038/s41586-021-04388-0. [DOI] [PubMed] [Google Scholar]
- 4.Brandal L.T., MacDonald E., Lamprini V., Ravlo T., Lange H., Naseer U., et al. Outbreak caused by the SARS-CoV-2 Omicron variant in Norway, November to December 2021. Euro Surveill. 2021;26 doi: 10.2807/1560-7917.ES.2021.26.50.2101147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pajon R., Doria-Rose N.A., Shen X., Schmidt S.D., O'Dell S., McDanal C., et al. SARS-CoV-2 Omicron variant neutralization after mRNA-1273 booster vaccination. N Engl J Med. 2022;386:1088–1091. doi: 10.1056/NEJMc2119912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Organization W.W.H. Classification of Omicron (B.1.1.529): SARS-CoV-2 variant of concern. https://www.who.int/news/item/26-11-2021-classification-of-omicron-(b.1.1.529)-sars-cov-2-variant-of-concern
- 7.Robilotti E.V., Whiting K., Lucca A., Poon C., Guest R., McMillen T., et al. Clin Infect Dis [Internet; 2021. Clinical and genomic characterization of SARS CoV-2 infections in mRNA vaccinated health care personnel in New York City. [cited]; ciab886. [Accessed 8 March 2022] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aslam A., Singh J., Robilotti E., Chow K., Bist T., Reidy-Lagunes D., et al. Severe acute respiratory syndrome coronavirus 2 surveillance and exposure in the perioperative setting with universal testing and personal protective equipment policies. Clin Infect Dis. 2021;73:e3013–e3018. doi: 10.1093/cid/ciaa1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Babady N.E., McMillen T., Jani K., Viale A., Robilotti E.V., Aslam A., et al. Performance of severe acute respiratory syndrome coronavirus 2 real-time RT-PCR tests on oral rinses and saliva samples. J Mol Diagn. 2021;23:3–9. doi: 10.1016/j.jmoldx.2020.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rosenberg E.S., Dorabawila V., Easton D., Bauer U.E., Kumar J., Hoen R., et al. Covid-19 vaccine effectiveness in New York State. N Engl J Med. 2022;386:116–127. doi: 10.1056/NEJMoa2116063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arbel R., Hammerman A., Sergienko R., Friger M., Peretz A., Netzer D., et al. BNT162b2 vaccine booster and mortality due to Covid-19. N Engl J Med. 2021;385:2413–2420. doi: 10.1056/NEJMoa2115624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bar-On Y.M., Goldberg Y., Mandel M., Bodenheimer O., Freedman L., Alroy-Preis S., et al. Protection against Covid-19 by BNT162b2 booster across age groups. N Engl J Med. 2021;385:2421–2430. doi: 10.1056/NEJMoa2115926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bar-On Y.M., Goldberg Y., Mandel M., Bodenheimer O., Freedman L., Kalkstein N., et al. Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med. 2021;385:1393–1400. doi: 10.1056/NEJMoa2114255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spitzer A., Angel Y., Marudi O., Zeltser D., Saiag E., Goldshmidt H., et al. Association of a third dose of BNT162b2 vaccine with incidence of SARS-CoV-2 infection among health care workers in Israel. JAMA. 2022;327:341–349. doi: 10.1001/jama.2021.23641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson M.G., Natarajan K., Irving S.A., Rowley E.A., Griggs E.P., Gaglani M., et al. Effectiveness of a third dose of mRNA vaccines against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of Delta and Omicron variant predominance–VISION Network, 10 states, August 2021–January 2022. MMWR Morb Mortal Wkly Rep. 2022;71:139–145. doi: 10.15585/mmwr.mm7104e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.U.H.S. Agency . 2022. COVID-19 vaccine surveillance report Week 4: 27 January 2022.https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1050721/Vaccine-surveillance-report-week-4.pdf [Google Scholar]
- 17.León T.M., Dorabawila V., Nelson L., Lutterloh E., Bauer U.E., Backenson B., et al. COVID-19 cases and hospitalizations by COVID-19 vaccination status and previous COVID-19 diagnosis —California and New York, May–November 2021. MMWR Morb Mortal Wkly Rep. 2022;71:125–131. doi: 10.15585/mmwr.mm7104e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.