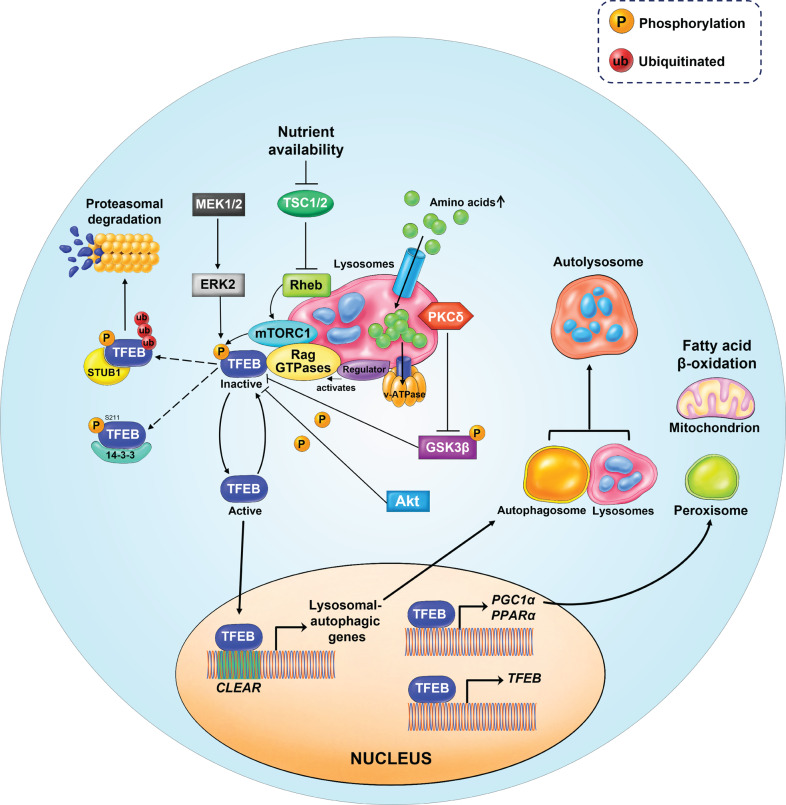
Fig. 5. Schematic model of TFEB upstream regulation by mTORC1.
In nutrient-rich conditions, for instance, amino stimulation, mTORC1 is recruited to the lysosomal membrane. Amino acids can freely cross the lysosomal membrane and accumulate inside the lysosomes. These amino acids are ‘sensed’ by the lysosomal lumen and signal to the Rag GTPases through the v-ATPase-Ragulator complex. Activated Rag GTPases recruit both TFEB and mTORC1 to the lysosomal membrane. Rheb promotes the activation of membrane-localized mTORC1. Active mTORC1 phosphorylates TFEB, thus causing its inactivation and inhibiting its nuclear translocation. In addition, TFEB can also be phosphorylated by ERK2, AKT, and GSK3β. Phosphorylated TFEB is recognized by STUB1, an E3-ligase, which then targets TFEB for proteasomal degradation. However, in nutrient- deprived conditions, TFEB becomes active and free from phosphorylation. Active TFEB then translocates to the nucleus, binds to the CLEAR motif, and increases the expression of genes involved in autophagy/lysosomal biogenesis. TFEB also binds to its own promoters and upregulates its expression through a self-regulatory feedback loop. TFEB is responsible for the transcriptional activation of PGC1α, the co-activator of the nuclear receptor peroxisome proliferator-activated receptor α (PPARα) and a key regulator of lipid metabolism and mitochondrial biogenesis.