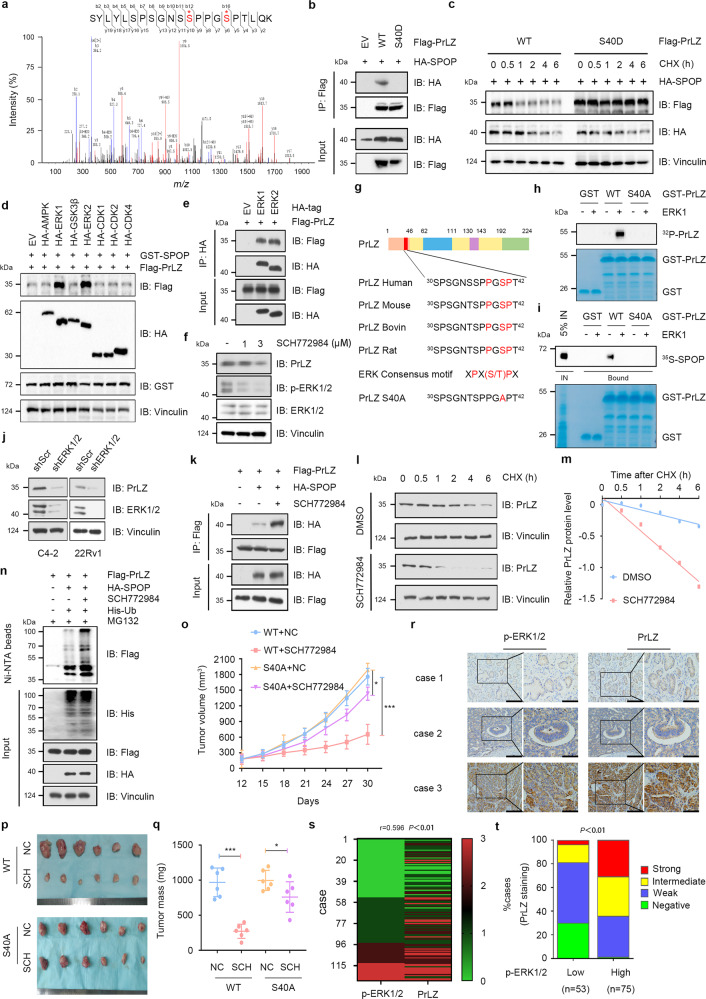
Fig. 5. ERK1/2-mediated phosphorylation of PrLZ at Ser40 stabilizes PrLZ through disrupting its binding with SPOP.
a Post-translational modifications of PrLZ identified by mass spectrometry (MS). Flag-PrLZ protein derived from 293 T cells were immunoprecipitated with anti-Flag antibody, separated by SDS–PAGE gel and subjected to in-gel digestion for MS. MS/MS spectrum of the PrLZ fragment from S30 to T42, m/z = 672.312 (Z = 2) was shown. b Immunoblot (IB) analysis of WCL and anti-Flag immunoprecipitates (IPs) derived from 293 T cells transfected with HA-SPOP, Flag-PrLZ WT, and Flag-PrLZ S40D mutant. 30 h post-transfection, cells were treated with 20 μM MG132 for 6 h before harvesting. EV, empty vector. WT, wild type. c IB analysis of WCL derived from 293 T cells transfected with HA-SPOP, Flag-PrLZ WT, and Flag-PrLZ S40D mutant. Where indicated, 100 μg/ml CHX was added for the indicated time period before harvesting. WT, wild type. d IB analysis of WCL derived from 293 T cells transfected with GST-SPOP, Flag-PrLZ WT, and indicated kinases constructs. EV, empty vector. e IB analysis of WCL and anti-HA IPs derived from 293 T cells transfected with Flag-PrLZ, HA-ERK1, and HA-ERK2. 30 h post-transfection, cells were treated with 20 μM MG132 for 6 h before harvesting. EV, empty vector. f IB analysis of WCL derived from C4-2 cells treated with SCH772984 (1 and 3 μM) for 24 h. g Sequence alignment of phosphorylation of PrLZ within the SPOP binding domain. h In vitro kinase assays showing that ERK1 phosphorylated recombinant PrLZ at Ser40. i ERK1-mediated phosphorylation of PrLZ hindered its interaction with SPOP in vitro. Autoradiograms showing recovery of 35S-labeled SPOP protein bound to the indicated GST-PrLZ fusion proteins (GST protein as a negative control). IN, input (5% as indicated). j IB analysis of WCL derived from C4-2 and 22Rv1 cells stably expressing shERK1/2 or shScr. Scr, Scramble. k IB analysis of WCL and anti-Flag IPs derived from 293 T cells transfected with Flag-PrLZ and HA-SPOP. 12 h post-transfection, cells were treated with 3 μM SCH772984 for additional 24 h before harvesting. Where indicated, 20 μM MG132 was added for 6 h before harvesting the cells. l IB analysis of WCL derived from C4-2 cells treated with or without 3 μM SCH772984. Where indicated, 100 μg/ml CHX was added for the indicated time period before harvesting. WT, wild type. m The PrLZ protein abundance in (l) was quantified by ImageJ and plotted as indicated. PrLZ bands were normalized to vinculin. n IB analysis of WCL and Ni-NTA pull-down products derived from PC-3 cells transfected with Flag-PrLZ, HA-SPOP and His-Ub. 12 h post-transfection, cells were treated with 3 μM SCH772984 for 24 h before harvesting. Where indicated, 20 μM MG132 was added for 6 h before harvesting the cells. o–q PC-3 cells stably expressing PrLZ-WT or PrLZ-S40A mutant were subcutaneously injected into nude mice with or without SCH772984 treatment (50 mg/kg, daily). Statistical analysis of the tumor volumes which were measured every three days and plotted individually (o). Subcutaneous xenograft tumors from different groups in PC-3 cells were dissected (p). Statistical analysis of the weights of the dissected xenografts tumors (q). n = 6 mice per experimental group, the results indicated the mean ± S.D. *P < 0.05 and ***P < 0.001. NC, non-specific control. WT, wild type. SCH, SCH772984. r Representative images of PCa patient samples stained for PrLZ and p-ERK1/2 expression by immunohistochemistry. Scale bar, left 200 μm, right 100 μm. s Correlation analysis of PrLZ and p-ERK1/2 expression in PCa patient samples. t Mann–Whitney test analysis of PrLZ expression in p-ERK1/2 low and high expression PCa patient samples.