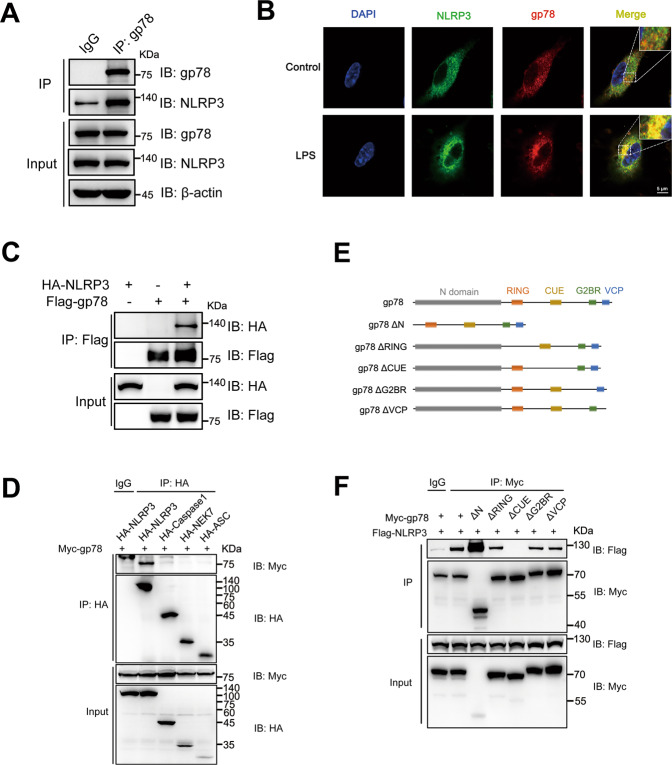
Fig. 3. gp78 interacts with NLRP3 via its CUE domain.
A Immunoblots of lysates from wild-type peritoneal macrophages primed with LPS (500 ng/ml) for 4 h immunoprecipitated with anti-gp78 antibody or anti-rabbit IgG and then immunoblotted with the indicated antibodies. B Confocal microscopy images of BMDMs with or without LPS showing co-localization of NLRP3 (green) with gp78 (red) (scale bar, 5 μm). C Immunoblots of lysates immunoprecipitated with anti-Flag antibody and then with the indicated antibodies from HEK293T cells 24 h after transfection with HA-tagged NLRP3 and Flag-tagged gp78. D HA-tagged NLRP3 inflammasome components were individually transfected into HEK293T cells with Myc-tagged gp78. 24 h after transfection, cell lysates were immunoprecipitated with anti-HA antibody or anti-Mouse IgG and then immunoblotted with the indicated antibodies. E Schematic diagram of gp78 and its truncated mutants. F Myc-tagged gp78 or its truncated mutants were individually transfected into HEK293T cells with Flag-tagged NLRP3. 24 h after transfection, cell lysates were immunoprecipitated with anti-Myc antibody or anti-Mouse IgG and then immunoblotted with the indicated antibodies. Data are representative of three independent experiments.