Abstract
There is a substantial burden of occupational health effects from heat exposure. We sought to assess the accuracy of estimated core body temperature (CBTest) derived from an algorithm that uses sequential heart rate and initializing CBT,1 compared with gastrointestinal temperature measured using more invasive ingestible sensors (CBTgi), among outdoor agricultural workers. We analyzed CBTest and CBTgi data from Washington State, USA, pear and apple harvesters collected across one work shift in 2015 (13,413 observations, 35 participants) using Bland Altman methods. The mean (standard deviation, range) CBTgi was 37.7 (0.4, 36.5–39.4)°C. Overall CBT bias (limits of agreement) was −0.14 (±0.76) °C. Biases ranged from −0.006 to −0.75 °C. The algorithm, which does not require the use of ingestible sensors, may be a practical tool in research among groups of workers for evaluating the effectiveness of interventions to prevent adverse occupational heat health effects.
Keywords: Agricultural workers, core body temperature, gastrointestinal temperature, heat-related illness, heat stress, physiological strain index
Introduction
There is a substantial burden of adverse health effects from heat exposure among working populations. Heat exposure can cause elevated core body temperatures, exertional heat stroke, which can be fatal, and other heat-related illnesses and is associated with traumatic injuries.2 Agricultural workers often perform physically demanding work in hot conditions and are at particularly high risk of adverse health effects from heat exposure. In the US, agricultural workers have 35 times the rate of heat-related deaths compared to workers in all other industries (rate ratio 35.2, 95% confidence interval [CI] 26.3–47.0), with a yearly average fatality rate of 3.1 per one million workers.3 In the agriculturally intensive US states of Washington and California, workers in the agriculture, forestry, and fishing (AFF) sector have higher rates of heat-related illness compared to other sectors.4,5 An international systematic review and meta-analysis of epidemiological studies found that, compared to other sectors, the agriculture sector had an increased risk of occupational injuries in the heat.6 Absent extensive restructuring of agriculture, this risk is not likely to decrease in the future, as the frequency and severity of extreme heat are projected to increase.7
Adverse health effects from heat exposure are preventable, and evaluations of the effectiveness of approaches that address risk factors at multiple levels (e.g., individual, coworker, employer, community, built environment, and policy levels) for agriculture are needed.8 A variety of promising heat stress prevention strategies exist,9–12 but few studies have evaluated the effectiveness of these approaches on large scales across multiple different settings. Research in California suggests an increased risk of heat-related illness even when farms follow California/Occupational Safety and Health Administration (OSHA) heat regulations.13 Practical and acceptable methods for assessing heat strain outcomes are needed for research that aims to evaluate the effectiveness of promising heat prevention approaches in the field.
There exist a variety of heat strain monitoring methods, each of which has different limitations and strengths.2 Though low-cost and practical, worker reporting of symptoms may be affected by recall bias or concerns about reporting, and awareness of heat-related illness symptoms may be compromised by the effect of heat on cognition.14 Measurement of heart rate alone may be difficult to interpret, as many factors influence heart rate including age, underlying health conditions, and medication use. Heart rate also fluctuates more rapidly than core body temperature, and determination of meaningful heart rate thresholds may need to consider time averaging of raw data and other criteria such as the relationship between heart rate and endurance time.15 Core body temperature (CBT) is a critical indicator of heat strain. The most accurate locations for measuring CBT are the rectum or the esophagus, but the invasiveness of these measurements limits their practicality outside the clinical setting. Gastrointestinal temperatures can be used to estimate CBT. Inert ingestible thermometer pills, which continuously transmit temperature to an external receiver as the capsule travels through the digestive tract, allow monitoring of CBTs in field settings. Ingestible CBT sensors have been used in some research studies to characterize heat strain.16–18 However, ingestible sensors are costly, are sensitive to food and fluid intake, and may not be indicated or acceptable for all people.19 Several methods have been proposed to predict CBT, but these methods rely on the concurrent assessment of several parameters, including height, weight, clothing, heart rate, respiratory rate, heat flux, accelerometry parameters, and skin temperature, which may be difficult to measure continuously during heavy physical work in the field.20–24
An ideal method for assessing heat strain for research would be reasonably accurate, involve as few parameters as possible, and would be cost efficient, practical, and acceptable to research participants.20 The US Army Research Institute of Environmental Medicine (USARIEM) developed a CBT estimation algorithm, based on one-minute heart rate measurements and baseline CBT, using an extended Kalman filter approach.1 The underlying rationale for the approach is that heart rate not only increases with physical work, from which approximately 80% of energy generated results in heat production, but heart rate also increases in the heat to support increased blood flow to the skin and subsequent evaporative heat loss.25 The USARIEM algorithm was developed using field data from 17 male US Army soldiers (mean [standard deviation] age = 23 [4] years) with CBTs ranging from 36–40 °C.1 The algorithm was evaluated on over 52,000 CBT measurements from nine laboratory and field studies with 82 male and one female volunteer (studies reporting mean ages of participants ranging from 22 to 28 years), a wide range of exertion levels (estimated as 200–1000 W), air temperatures (9–47 °C), relative humidities (9–95%), hydration states, clothing ensembles, and acclimatization states against CBT measured by rectal probe, thermometer pill suppository, and ingested thermometer pills (overall bias −0.03 ± 0.32 °C, 95% limit of agreement (LoA) of ±0.63 °C).25 The algorithm has since been updated to incorporate a sigmoid equation to better correct lower CBT estimates (ECTemp™),26 and validation among 21 male Royal Marines compared to gastrointestinal temperatures indicated a bias of −0.10 ± 0.37 °C for those with progressively increasing CBTs over 40 °C and a bias of 0.34 ± 0.40 °C among the other participants.27 However, there have been limited algorithm evaluations outside of military settings or in samples that include female or older workers.
The overall goal of this study was to assess the accuracy of the algorithm among civilian outdoor agricultural workers in the field. We aimed to describe and compare the bias and LoA of estimated CBT derived from the algorithm with gastrointestinal temperatures measured using ingestible sensors, in a field setting among Washington State, US outdoor agricultural workers. We hypothesized that while the algorithm may be less accurate than reported in more controlled military settings, the estimated CBT derived from the algorithm may be a useful method for research that evaluates heat stress prevention approaches.
Materials and methods
We conducted a secondary analysis of data from a 2015 field study that aimed to assess potential mechanisms of the relationship between heat exposure and traumatic injuries28 and productivity18 among Washington State, US, agricultural workers. In the present analysis, we described and compared estimates of CBT from ingestible gastrointestinal temperature sensors to those from the algorithm using Bland Altman methods.
Study population, recruitment, and sampling
As previously described, we recruited a convenience sample of adult (age 18 or older) tree fruit workers from Central/Eastern Washington State through University of Washington (UW) Pacific Northwest Agricultural Safety and Health (PNASH) Center contacts.18,28 Forty-six (34 pear and 12 apple) harvesters from six (five pear and one apple) orchards were recruited to participate for one work shift each during the summer of 2015. The mean (standard deviation) total kilograms harvested per hour was 341 (84).18 We assumed that all participants were acclimatized based on their responses to survey questions about duration of work during the season.
Pear harvesters participated in August and apple harvesters participated in September, in accordance with usual harvest timing. We included both August and September to enhance variability in heat exposure. In Washington State agriculture, workers’ compensation claims for heat-related illness occur more frequently in August than September, but heat-related illness has been reported in September.29 We monitored a maximum of four harvesters daily. All participants were paid by the piece (amount harvested). In Central/Eastern Washington, agricultural workers are largely Spanish speaking seasonal immigrant workers and migrant workers.30 The climate in Central/Eastern Washington is notable for hotter and more arid summers, compared to Western Washington.31 Participants provided informed consent prior to participation, and all study procedures were approved by the UW Institutional Review Board.
Gastrointestinal and estimated core body temperatures and physiological strain index
Workers’ gastrointestinal temperatures were measured every 20 seconds using CorTemp™ FDA registered and cleared (510 K, No. 880639) inert ingestible thermometer capsules (HQ, Inc; Palmetto, FL), which were ingested with lukewarm water 30 minutes, on average, before the start of the work-shift. Workers’ heart rates were assessed every 20 seconds using Polar® chest band monitors (Polar, Inc; Lake Success, NY). Baseline body temperatures were measured using tympanic thermometers (Braun; Kronberg, Germany) at the start of the work-shift. Workers were not able to ingest the gastrointestinal sensor the night before the work-shift, and we were not able to measure participants’ baseline CBTs using gastrointestinal sensors due to work and logistical considerations.
Gastrointestinal temperature (CBTgi) and heart rate data were wirelessly transmitted to data recorders worn by workers and subsequently downloaded onto research computers for analysis. We excluded CBTgi measurements below 34 °C and above 42 °C and heart rate measurements below 40 beats per minute (bpm) or above 200 bpm, as these values were considered outside of the physiologically expected range. Five-point rolling medians of the data were calculated, and missing data were imputed as the median of the five values immediately before and five values immediately after the missing value. We did not impute consecutive missing values that exceeded one minute, and we excluded these points from the analysis. One-minute average values were computed, and data were trimmed to remove the first 30 minutes of work, given reported algorithm conversation times25 and CBTgi equilibration times.32
We derived estimated CBTs (CBTest) from the algorithm,1 using baseline CBT and heart rate data. We computed CBTest using a default baseline CBT of 37.1 °C25 and using a baseline CBT estimated from the morning pre-work tympanic temperature +0.27 °C to account for differences between tympanic temperature and CBT.33
We calculated the physiological strain index (PSI) to compare PSI computed using CBTgi (PSIgi) with PSI computed using CBTest (PSIest). We calculated PSI using the equation PSI = 5*[(Tx −T0)/(39.5–T0)] + 5*[(HRx −HR0)/(180–HR0)], where Tx is the core temperature at time x, T0 is baseline core temperature, HRx is heart rate at time x, and HR0 is baseline heart rate.34 The higher the PSI, the higher the heat strain.34 Resting heart rate was not consistently collected, so we imputed baseline heart rate (T0) using a regression equation relating measured resting heart rate to the average of the first 5 minutes of heart rate as described in the Supplementary Material: Additional Methods.
Workplace and individual characteristics
We measured Wet-Bulb Globe Temperatures (WBGTs) using a hand-held digital WBGT meter (Extech HT30 WBGT Meter, Extech Instruments; Nashua, NH) near individual workers during the work shift approximately every 1–3 hours. Research staff recorded work and break times, clothing observations, and activity level observations on standardized data sheets.
We also assessed individual and workplace characteristics using an audio computer-assisted self-interview survey instrument, which has been assessed for reliability and validity among farmworkers, as previously described.28,35 We measured height and weight the day the worker participated in the field study to calculate body mass index (BMI [kg/m2]).36
Analyses
We used descriptive statistics to characterize workplace and individual factors. We excluded sample participants if they had substantial amounts of missing data, more than ten minutes of missing consecutive heart rate and CBT data, or CBTgi dipping below 36.5 °C. The quadratic version of the algorithm does not adequately predict CBT under 36.0 °C.26 Eleven participants (seven pear harvesters and four apple harvesters) of the original 46 were excluded, and a total of 35 participants were included in the analyses.
We created time-series plots of heart rate, CBTgi, and CBTest. We assessed the number of participants with more than three CBT values greater than 38.5 °C and 38.0 °C. A CBT above 38.5 °C for acclimatized individuals and 38.0 °C for unacclimatized individuals may indicate physiological heat strain.37 Though we did ask about the timing of agricultural work during the season and the number of days worked in the past week in the survey, we did not measure acclimatization status of individual participants and therefore assessed both thresholds. We used the Bland-Altman method,38 with CBTest and PSIest as ‘estimated’ values and CBTgi and PSIgi as ‘observed’ values, to assess the accuracy of the algorithm. We reported bias (mean of differences, observed-estimated), LoA (bias ± 1.96*standard deviation of differences), root mean square error (RMSE = (sqrt [sum of the square of differences/sample size]), and mean absolute error (MAE = sum of the absolute value of the difference between observed values and the mean of the estimated values/sample size) for the entire sample and separately for apple (September) and pear (August) workers, males and females, age under 50 years and 50 or greater years, and for individual participants. We created scatter plots of observed versus estimated values, Bland-Altman plots (differences versus mean of observed and estimated values), and histograms of differences, bias, RMSE, and MAE to describe accuracy. We conducted analyses using both: (1) the default 37.1 °C; and (2) aural temperature +0.27 °C as the baseline temperature for the algorithm. Analyses were conducted using RStudio Server Version 1.4.1717 (R Foundation, Vienna, Austria)39 and the ‘blandr’ package version 0.5.1.40
Results
Work and individual characteristics
The mean (standard deviation, range) maximum work-shift WBGT was 27.3 (3.3, 22.0–33.1) °C in August and 21.6 (1.7, 19.0–22.9) °C in September. Workers started work on average around 6 a.m. and finished around 1 p.m.28 Work times averaged 6.8 (standard deviation 1.5) hours, and most workers took one break that averaged about 20 minutes in the midmorning to eat lunch.28 All workers were observed to be performing moderate metabolic rate (300 Watt) work activities with 75–100% allocation of work in a work/recovery cycle.37 In general, participants wore short or long-sleeved shirts underneath hooded sweatshirts or button-down shirts and long pants.28
Individual and workplace characteristics of the 35 participants included in the Bland-Altman analyses are shown in Table S1 in the Supplementary Material. There were only one to three reports each of having been told by a healthcare provider about having diabetes mellitus, high blood pressure, heart disease, or overweight/obesity. Only two (6%) participants reported taking cardiovascular medications. The mean (standard deviation) BMI of participants was 28.1 (4.3) kg/m2. Characteristics of excluded participants are described in the Supplementary Material.
The mean (standard deviation, range) gastrointestinal temperature was 37.7 (0.4, 36.5–39.4) °C. Sixty-nine percent and 17% of participants had at least three CBTgi measurements over 38.0 °C and 38.5 °C, respectively. Seventy-one percent and 34% of participants had at least three CBTest measurements over 38.0 °C and 38.5 °C, respectively. Individual tracings of heart rate, CBTest, and CBTgi are shown in Figure S1 in the Supplementary Material. There were observable consistent differences between the CBTest and CBTgi, with CBTest higher than CBTgi, on average.
Accuracy assessment
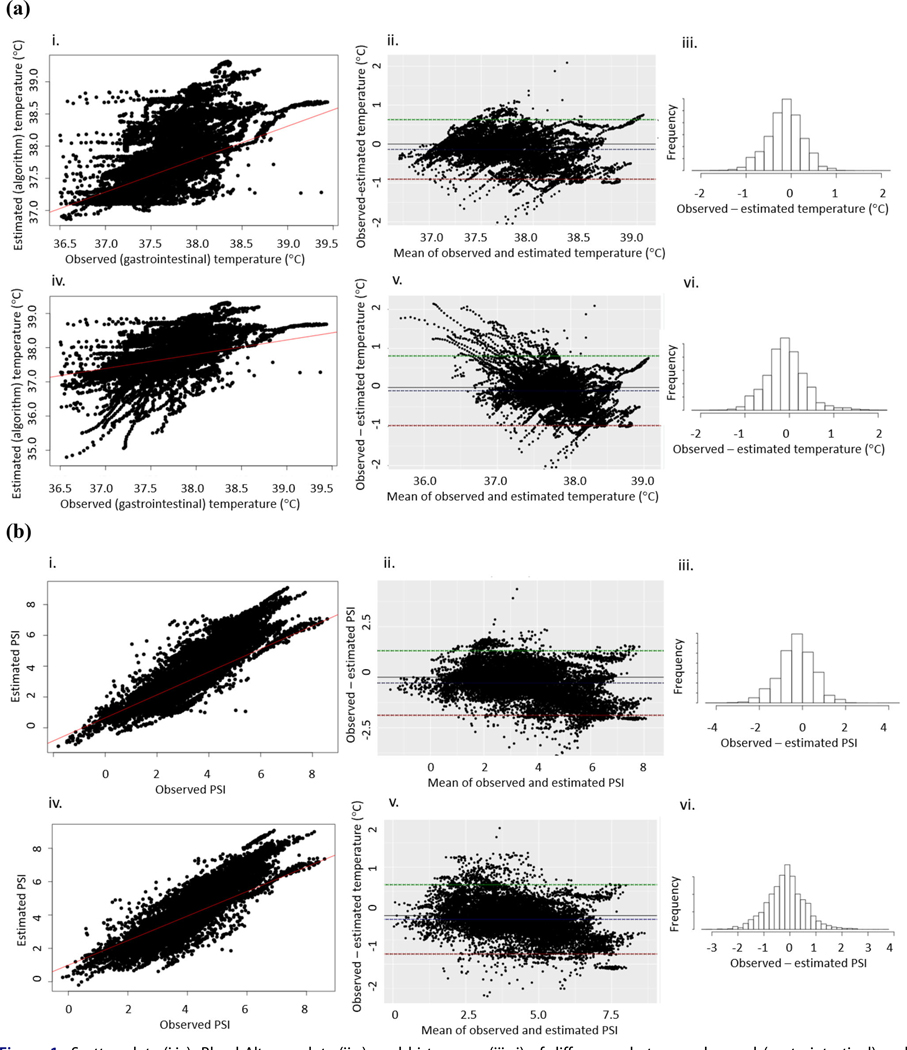
Table 1 reports bias and 95% LoA, using a baseline CBT of 37.1 °C and aural temperature +0.27 °C, for CBT and PSI for participants included in the analysis. The overall CBT and PSI biases were −0.14 °C with LoA of ±0.76 °C and −0.29 with LoA of ±1.59, respectively, using the default 37.1 °C baseline CBT. Scatter plots, Bland-Altman plots, and histograms of differences between CBT and PSI derived from observed (gastrointestinal) and estimated (algorithm) CBTs are shown in Figure 1a and 1b, respectively. The overall bias was negative, indicating that the estimated (CBTest) values were, on average, larger than the observed (CBTgi) values. However, observed (CBTgi) values were generally higher than estimated (CBTest) values for lower mean (of estimated and observed) CBT values using the aural temperature +0.27 °C baseline CBT (Figure 1a[v]). For all participants, bias was lower using the aural temperature +0.27 °C (CBT bias −0.09, 95% LoA ± 0.90 °C; PSI bias −0.15, 95% LoA ± 1.42) versus the 37.1 °C baseline. RMSEs and MAEs were not substantially different from one another.
Table 1.
Bias, limits of agreement (LoA), root mean square error (RMSE), and mean absolute error (MAE) between observed (gastrointestinal) and estimated (algorithm) (a) core body temperature (°C) and (b) physiological strain index (PSI).
| (a) Baseline temperature |
Bias |
LoA |
RMSE |
MAE |
||||
|---|---|---|---|---|---|---|---|---|
| 37.1 °C | aural +0.27 °C | 37.1 °C | aural +0.27 °C | 37.1 °C | aural +0.27 °C | 37.1 °C | aural +0.27 °C | |
|
| ||||||||
| All (N = 35) | −0.14 | −0.09 | ±0.76 | ±0.90 | 0.41 | 0.46 | 0.32 | 0.35 |
| Males (n = 31) | −0.16 | −0.10 | ±0.73 | ±0.87 | 0.41 | 0.46 | 0.31 | 0.34 |
| Females (n = 4) | −0.005 | 0.02 | ±0.93 | ±1.00 | 0.48 | 0.52 | 0.41 | 0.43 |
| Age < 50 years (n = 25) | −0.17 | −0.12 | ±0.65 | ±0.79 | 0.37 | 0.42 | 0.29 | 0.32 |
| Age ≥ 50 years (n = 10) | −0.04 | 0.03 | ±0.99 | ±1.11 | 0.51 | 0.57 | 0.40 | 0.45 |
| August pear harvesters (n = 27) | −0.22 | −0.16 | ±0.72 | ±0.86 | 0.43 | 0.47 | 0.32 | 0.35 |
| September apple harvesters (n = 8) | 0.12 | 0.18 | ±0.68 | ±0.78 | 0.37 | 0.44 | 0.31 | 0.35 |
| (b) | ||||||||
| All (N = 35) | −0.29 | −0.15 | ±1.59 | ±1.42 | 0.86 | 0.74 | 0.67 | 0.57 |
| Males (n = 31) | −0.32 | −0.17 | ±1.52 | ±1.37 | 0.84 | 0.72 | 0.64 | 0.54 |
| Females (n = 4) | −0.0099 | −0.050 | ±1.94 | ±1.79 | 0.99 | 0.91 | 0.85 | 0.77 |
| Age < 50 years (n = 25) | −0.36 | −0.22 | ±1.35 | ±1.31 | 0.78 | 0.70 | 0.60 | 0.53 |
| Age ≥ 50 years (n = 10) | −0.09 | 0.03 | ±2.07 | ±1.66 | 1.06 | 0.85 | 0.84 | 0.68 |
| August pear harvesters (n = 27) | −0.45 | −0.28 | ±1.50 | ±1.40 | 0.89 | 0.77 | 0.67 | 0.58 |
| September apple harvesters (n = 8) | 0.25 | 0.26 | ±1.42 | ±1.19 | 0.77 | 0.67 | 0.64 | 0.54 |
Figure 1.
Scatter plots (i,iv), Bland-Altman plots (ii,v), and histograms (iii,vi) of differences between observed (gastrointestinal) and estimated (algorithm) (a) core body temperatures (CBTs) and (b) physiological strain index (PSI) based on observed (gastrointestinal) and estimated (algorithm) CBTs using: (i–iii) default 37.1 °C; and (iv–vi) estimated baseline CBT (aural temperature +0.27 °C). Scatter plots include a best fit regression line; Bland-Altman plots include dotted lines representing bias and limits of agreement.
Table 1 reports bias, 95% LoA, RMSE, and MAE using a baseline CBT of 37.1 °C and aural temperature + 0.27 ° for CBT and PSI, respectively, stratified by gender, age, and crop/month. Using 37.1 °C baseline, bias was lower for females (−0.005, LoA of ±0.93 °C) than males (−0.16, LoA of ±0.73 °C) and for those 50 years or older −0.04, LoA ±0.99) compared to younger than 50 years (−0.17, ±LoA −0.65). Bias was −0.22 with LoA of ±0.72 °C for pear harvesters but was 0.12 with LoA of ±0.68 °C for apple harvesters.
Table S2 and Figures S2 & S3 in the Supplementary Material show bias, 95% LoA, RMSE, and MAE for CBT by participant using the 37.1 °C baseline CBT and histograms of individual bias and RMSE, respectively. Though the overall bias was relatively low (−0.14 °C), biases ranged in magnitude from 0.006 to 0.75 °C (Table S2). In a post-hoc analysis, we examined characteristics of the five participants with biases of 0.50 °C or larger magnitudes in the negative direction (‘high bias’ participants). The age range of high bias participants ranged from 19 to 63 years with a mean of 44.8 years. The mean (range) BMI of high bias participants was 27.6 (20.1–33.4) kg/m2. Two of the high bias participants reported a history of hypertension and taking anti-hypertensive medications. All high bias participants harvested pears, and only one of the five high bias participants was female. High bias participants worked in conditions of mean (range) WBGT 27.5 (22.0–31.6) °C.
Discussion
We evaluated, among Washington State agricultural workers in the field (35 participants, 13,413 observations), an algorithm developed in a military setting that is used to estimate CBT based on sequential heart rate measurements and baseline CBT.1 In our study, in which we compared gastrointestinal temperatures using ingestible CBT sensors to estimated CBTs from the algorithm, we found a reasonable overall accuracy of the algorithm. Our study demonstrates the promise of this algorithm in research evaluating the effectiveness of interventions to prevent adverse occupational heat health effects. However, inter-individual variability in bias suggests that caution should be applied when evaluating individual workers.
We found an overall bias for CBT of −0.14 °C using a baseline CBT of 37.1 °C. This is a higher magnitude of bias than previously reported in primarily young male military populations (biases generally smaller than 0.10 °C in magnitude).25,26,41,42 The analysis sample used to develop the estimated CBT algorithm (all male US Army soldiers, mean age 23 years)1 differed from the participant sample included in this study (11% female, mean age 39 years, largely Latinx). However, the work rate, clothing ensemble, and environmental conditions observed in this study fall within the range of conditions in which the algorithm has been evaluated.25,41 In our study, bias was lower for females than males, though with larger limits of agreement, consistent with the small sample size of females in our study. Still, our study contains a larger proportion of females than other algorithm field validation studies of which we are aware. Using the baseline aural temperature +0.27 °C, the CBT bias was −0.09 °C, which was lower than when using a standard baseline value and raises the question of improved accuracy using a measured baseline value.
The overall bias for CBT and PSI was negative, indicating that estimated values were, on average, larger than the observed (gastrointestinal) values. However, differences in observed and estimated CBT values were generally positive (observed values higher than estimated values) and larger for lower mean CBT values using the aural temperature +0.27 °C baseline CBT (Figure 1a[v]). This could indicate measurement bias with the baseline CBT estimation approach, for example with falsely low aural temperatures in cooler early morning conditions. However, there is less concern for underestimation of CBT early in the work-shift when the CBT is lower, as workers are less likely to exceed safe CBTs until later in the shift. RMSEs and MAEs were not substantially different, indicating minimal large differences between observed and expected values, which would be weighted more heavily in the RMSE calculation. Overall, the negative bias for the entire sample is health conservative, with estimated values slightly higher than observed values, on average.
The overall bias for PSI was −0.29 when 37.1 °C was used as the baseline temperature and lower using the aural temperature + 0.27 °C baseline. As originally conceptualized by Moran, PSI is categorized into five physiological strain groups, each with a range of two units, within a total ran–e of 1–10 units. The magnitude of the overall bias for PSI in our study is only 15% of the width of a PSI strain category, and the magnitude of the LoA was also less than two units.
Gastrointestinal temperature is not a perfect gold standard. The accuracy of the gastrointestinal temperature sensor is ±0.1 °C,43 which is similar in magnitude to the overall bias observed among our participants. Among studies comparing rectal and esophageal estimates of CBT, the magnitude of bias ranged from 0.01 to 0.35 °C with a weighted mean LoA of ±0.58 °C.1 A LoA of ±0.78 °C has been reported comparing rectal temperature and pulmonary arterial temperature,44 which is similar to our LoA of ±0.76 °C. This LoA is also within the range found by Brauer et al. (±0.82 °C) for comparison of rectal and esophageal estimates of CBT.45 Overall, the algorithm performance was within the range of accuracy seen with other estimates of CBT, compared to more invasive gold standards.
Though the overall CBT bias was relatively low (−0.14 °C), biases among individuals ranged from −0.006 to −0.75 °C (Table S2). Our post-hoc analysis did not reveal any obvious characteristics associated with high bias participants. However, it should be noted that certain anti-hypertensive medications lower heart rate, though we might expect this to result in estimated CBTs that are lower than observed CBTs. Though the algorithm may be useful for research among groups of workers, our results suggest that additional individual monitoring and assessment should be considered when making health and safety decisions for individuals in a practice setting.
We observed CBTgi exceeding 38.5 °C among 17% of participants.37 This is consistent with reports among agricultural workers in other US States. For example, among 221 nursery, fernery, and crop workers over three summer workdays in Florida, 16% of participants exceeded a gastrointestinal temperature of 38.5 °C on at least one day.46 Compared to CBTgi, CBTest exceeded 38.5 °C in a larger proportion (34%) of participants, suggesting that the algorithm may be more likely to detect potential heat strain.
Our study is one of the few studies that we are aware of to evaluate the algorithm in a civilian field setting among a more heterogeneous group of participants that included females and older Latinx agricultural workers, compared to other algorithm validation studies focused on young male military participants. However, our study has several important limitations. First, it is difficult to discern the underlying reasons why participants met our exclusion criteria of missing data and measured gastrointestinal temperatures below 36.5 °C. We did note a larger prevalence of chronic disease and anti-hypertensive medication use among excluded participants. Caution should be used when assessing heat strain among participants with underlying chronic disease and medication use that may affect measurements. In a practice setting, preplacement medical screening should be considered to identify and provide appropriate guidance and management to those with an increased risk of adverse health effects from heat.37 Second, we were not able to identify all factors associated with variability in accuracy. Future studies should endeavor to explore additional factors and develop methods tailored to high-risk occupational groups, including agricultural workers. In addition, as previously mentioned, gastrointestinal temperature is not a perfect gold standard. We do not have information on how frequently eating and drinking occurred during the work-shift and what temperature the fluids were, which is most relevant to the gastrointestinal temperature estimates, and this could affect accuracy estimates. Third, there were only four female participants in the study. However, there were more females in our study than prior published studies. Future studies with a larger number of females should be performed. Finally, our results may not be generalizable to workers in other settings and industries.
Conclusion
Given the large burden of heat stress among agricultural workers, additional research that evaluates the effectiveness of promising heat prevention approaches in field settings is needed. In our study population of outdoor agricultural workers, an algorithm based on sequential heart rate and initializing CBT1 demonstrated reasonable accuracy among groups of participants. However, inter-individual variability in bias observed in this study suggests that caution should be used when evaluating individual workers using this approach. The algorithm, which does not require the use of ingestible sensors, can be a practical tool in research evaluating the effectiveness of interventions to prevent adverse occupational heat health effects among groups of workers.
Supplementary Material
Acknowledgments
The authors would like to thank all participating workers and growers for their participation. The authors would like to acknowledge Maria Negrete and Jose Carmona for their assistance with data collection and Susan Wu for her assistance with data analysis.
Funding
This work was supported by the Centers for Disease Control and Prevention/National Institute for Occupational Safety and Health under Grant Numbers 5K01OH010672-02 to J.T.S. and 5U54OH007544-17.
Footnotes
Disclosure statement
The findings and conclusions in this paper are those of the authors and do not necessarily represent the official position of the National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention. Its contents, including any opinions and/or conclusions expressed, are solely those of the authors. No potential competing interest was reported by the authors.
Supplemental data for this article is available online at https://doi.org/10.1080/19338244.2022.2033672
Data availability statement
De-identified data and statistical code will be made available upon reasonable request to the corresponding author.
References
- 1.Buller MJ, Tharion WJ, Cheuvront SN, et al. Estimation of human core temperature from sequential heart rate observations. Physiol Meas. 2013; 34(7):781–798. doi: 10.1088/0967-3334/34/7/781. [DOI] [PubMed] [Google Scholar]
- 2.Jacklitsch B, Williams WJ, Musolin K, Coca A, Kim J-H, Turner N. 2016. NIOSH criteria for a recommended standard: Occupational exposure to heat and hot environments. Cincinnati, OH: NIOSH. https://www.cdc.gov/niosh/docs/2016-106/pdfs/2016-106.pdf?id=10.26616/NIOSHPUB2016106. Accessed 20 Sept 2021. [Google Scholar]
- 3.Gubernot DM, Anderson GB, Hunting KL. Characterizing occupational heat-related mortality in the United States, 2000–2010: an analysis using the census of fatal occupational injuries database. Am J Ind Med. 2015;58(2):203–211. doi: 10.1002/ajim.22381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Heinzerling A, Laws RL, Frederick M, et al. Risk factors for occupational heat-related illness among California workers, 2000–2017. Am J Ind Med. 2020; 63(12):1145–1154. doi: 10.1002/ajim.23191. [DOI] [PubMed] [Google Scholar]
- 5.Hesketh M, Wuellner S, Robinson A, Adams D, Smith C, Bonauto D. Heat related illness among workers in Washington State: A descriptive study using workers’ compensation claims, 2006–2017. Am J Ind Med. 2020; 63(4):300–311. doi: 10.1002/AJIM.23092. [DOI] [PubMed] [Google Scholar]
- 6.Binazzi A, Levi M, Bonafede M, et al. Evaluation of the impact of heat stress on the occurrence of occupational injuries: Meta-analysis of observational studies. Am J Ind Med. 2019;62(3):233–243. doi: 10.1002/ajim.22946. [DOI] [PubMed] [Google Scholar]
- 7.IPCC. 2014. Climate change 2014 synthesis report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change 1–151. https://www.ipcc.ch/report/ar5/syr/. Accessed 20 Sept 2021.
- 8.Spector JT, Masuda YJ, Wolff NH, Calkins M, Seixas N. Heat exposure and occupational injuries: Review of the literature and implications. Curr Environ Health Rep. 2019;6(4):286–296. doi: 10.1007/s40572-019-00250-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chicas R, Xiuhtecutli N, Elon L, et al. Cooling interventions among agricultural workers: A pilot study. Workplace Health Saf. 2021;69(7):315–322. doi: 10.1177/2165079920976524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krenz J, Santos E, Torres E, et al. The multi-level heat education and awareness tools [HEAT] intervention study for farmworkers: Rationale and methods. Contemp Clin Trials Commun. 2021;22:100795. doi: 10.1016/J.CONCTC.2021.100795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lam M, Krenz J, Palmandez P, et al. Identification of barriers to the prevention and treatment of heat-related illness in Latino farmworkers using activity-oriented, participatory rural appraisal focus group methods. BMC Public Health. 2013;13:1004 doi: 10.1186/1471-2458-13-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wegman DH, Apelqvist J, Bottai M, Work Health and Efficiency (WE) Program Working Group, et al. Intervention to diminish dehydration and kidney damage among sugarcane workers. Scand J Work Environ Health. 2018;44(1):16–24. doi: 10.5271/sjweh.3659. [DOI] [PubMed] [Google Scholar]
- 13.Langer CE, Mitchell DC, Armitage TL, et al. Are Cal/OSHA regulations protecting farmworkers in California from heat-related illness? J Occup Environ Med. 2021; 63(6):532–539. doi: 10.1097/JOM.0000000000002189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakata H, Kakigi R, Shibasaki M. Effects of passive heat stress and recovery on human cognitive function: an ERP study. PLoS One. 2021;16(7):e0254769. doi: 10.1371/journal.pone.0254769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bernard TE, Kenney WL. Rationale for a personal monitor for heat strain. Am Ind Hyg Assoc J. 1994; 55(6):505–514. doi: 10.1080/15428119491018772. [DOI] [PubMed] [Google Scholar]
- 16.Mitchell DC, Castro J, Armitage TL, et al. Recruitment, methods, and descriptive results of a physiologic assessment of Latino farmworkers: The California Heat Illness Prevention Study. J Occup Environ Med. 2017;59(7):649–658. doi: 10.1097/JOM.0000000000000988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mix JM, Elon L, Mac VV, et al. Physical activity and work activities in Florida agricultural workers. Am J Ind Med. 2019;62(12):1058–1067. doi: 10.1002/AJIM.23035. [DOI] [PubMed] [Google Scholar]
- 18.Quiller G, Krenz J, Ebi K, et al. Heat exposure and productivity in orchards: implications for climate change research. Arch Environ Occup Health. 2017; 72(6):313–316. doi: 10.1080/19338244.2017.1288077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wilkinson D, Carter J, Richmond V, Blacker S, Rayson M. The effect of cool water ingestion on gastrointestinal pill temperature. Med Sci Sports Exerc. 2008; 40(3):523–528. doi: 10.1249/MSS.0B013E31815CC43E. [DOI] [PubMed] [Google Scholar]
- 20.Buller MJ, Delves SK, Fogarty AL, Veenstra BJ. On the real-time prevention and monitoring of exertional heat illness in military personnel. J Sci Med Sport; Online Ahead of Print. 2021;24(10):975–981.) doi: 10.1016/j.jsams.2021.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Eggenberger P, MacRae B, Kemp S, Bürgisser M, Rossi R, Annaheim S. Prediction of core body temperature based on skin temperature, heat flux, and heart rate under different exercise and clothing conditions in the heat in young adult males. Front Physiol. 2018;9:1780. doi: 10.3389/FPHYS.2018.01780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niedermann R, Wyss E, Annaheim S, Psikuta A, Davey S, Rossi R. Prediction of human core body temperature using non-invasive measurement methods. Int J Biometeorol. 2014;58(1):7–15. doi: 10.1007/S00484-013-0687-2. [DOI] [PubMed] [Google Scholar]
- 23.Richmond V, Davey S, Griggs K, Havenith G. Prediction of core body temperature from multiple variables. Ann Occup Hyg. 2015;59(9):1168–1178. doi: 10.1093/ANNHYG/MEV054. [DOI] [PubMed] [Google Scholar]
- 24.Yokota M, Berglund L, Cheuvront S, et al. Thermoregulatory model to predict physiological status from ambient environment and heart rate. Comput Biol Med. 2008;38(11–12):1187–1193. doi: 10.1016/J.COMPBIOMED.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 25.Showers K, Hess A, Telfer B. 2016. Validation of core temperature estimation algorithm. Project Report PSM-4. Lexington, MA: Lincoln Laboratory of the Massachusetts Institute of Technology. https://apps.dtic.mil/dtic/tr/fulltext/u2/1034034.pdf. Accessed 20 Sept 2021. [Google Scholar]
- 26.Looney DP, Buller MJ, Gribok AV, et al. Estimating resting core temperature using heart rate. J Meas Physical Behaviour. 2018;1(2):79–86. doi: 10.1123/jmpb.2017-0003. [DOI] [Google Scholar]
- 27.Buller MJ, Davey T, Fallowfield JL, Montain SJ, Hoyt RW, Delves SK. Estimated and measured core temperature responses to high-intensity warm weather military training: Implications for exertional heat illness risk assessment. Physiol Meas. 2020;41(6):065011. doi: 10.1088/1361-6579/ab934b. [DOI] [PubMed] [Google Scholar]
- 28.Spector JT, Krenz J, Calkins M, et al. Associations between heat exposure, vigilance, and balance performance in summer tree fruit harvesters. Appl Ergon. 2018;67:1–8. doi: 10.1016/j.apergo.2017.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spector JT, Krenz J, Rauser E, Bonauto DK. Heat-related illness in Washington State agriculture and forestry sectors. Am J Ind Med. 2014;57(8):881–895. doi: 10.1002/ajim.22357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.WA Employment Security Department. 2013. Agricultural workforce in Washington State, https://esd.wa.gov/labormarketinfo/ag-employment-and-wages. Accessed 20 Sept 2021.
- 31.Western Regional Climate Center. 2014. Climate of Washington. http://www.wrcc.dri.edu/narratives/WASHINGTON.htm. Accessed 20 Sept 2021.
- 32.Domitrovich JW, Cuddy JS, Ruby BC. Core-temperature sensor ingestion timing and measurement variability. J Athl Train. 2010;45(6):594–600. doi: 10.4085/1062-6050-45.6.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huggins R, Glaviano N, Negishi N, Casa DJ, Hertel J. Comparison of rectal and aural core body temperature thermometry in hyperthermic, exercising individuals: a meta-analysis. J Athl Train. 2012;47(3): 329–338. doi: 10.4085/1062-6050-47.3.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moran D, Shitzer A, Pandolf K. A physiological strain index to evaluate heat stress. Am J Physiol. 1998; 275(l):R129–34. doi: 10.1152/ajpregu.1998.275.1.R129. [DOI] [PubMed] [Google Scholar]
- 35.Spector JT, Krenz J, Blank KN. Risk factors for heat-related illness in Washington crop workers. J Agromedicine. 2015;20(3):349–359. doi: 10.1080/1059924X.2015.1047107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention. 2014. How is BMI calculated and interpreted? About BMI for adults. Centers for Disease Control and Prevention. http://www.cdc.gov/healthyweight/assessing/bmi/adult_bmi/index.html#Interpreted. Accessed 20 Sept 2021. [Google Scholar]
- 37.ACGIH. Heat Stress and Strain: TLV® Physical Agents. Cincinnati, OH: American Conference of Governmental Industrial Hygienists; 2015. ISBN 9781-607260-77-6. [Google Scholar]
- 38.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;327(8476):307–310. doi: 10.1016/S0140-6736(86)90837-8. [DOI] [PubMed] [Google Scholar]
- 39.R Development Core Team. 2011. R: A language and environment for statistical computing. R Foundation for Statistical Computing, http://www.r-project.org. Accessed 20 Sept 2021.
- 40.Datta D 2018. blandr: Bland-Altman Method Comparison, cran.r-project.org. https://cran.r-project.org/web/packages/blandr/index.html. Accessed 5 August 2021.
- 41.Buller M, Tharion W, Duhamel C, Yokota M. Realtime core body temperature estimation from heart rate for first responders wearing different levels of personal protective equipment. Ergonomics. 2015;58(11): 1830–1841. doi: 10.1080/00140139.2015.1036792. [DOI] [PubMed] [Google Scholar]
- 42.Hunt AP, Buller MJ, Maley MJ, Costello JT, Stewart IB. Validity of a noninvasive estimation of deep body temperature when wearing personal protective equipment during exercise and recovery. Mil Med Res. 2019;6(1):20. doi: 10.1186/s40779-019-0208-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.HQInc. CorTempTM core body temperature monitoring system user manual. Palmetto, FL; 2015. http://www.heatstress.nl/downloads/getDownload/id/7. Accessed 20 Sept 2021. [Google Scholar]
- 44.Lefrant J-Y, Muller L, de La Coussaye JE, et al. Temperature measurement in intensive care patients: comparison of urinary bladder, oesophageal, rectal, axillary, and inguinal methods versus pulmonary artery core method. Intensive Care Med. 2003;29(3): 414–418. doi: 10.1007/s00134-002-1619-5. [DOI] [PubMed] [Google Scholar]
- 45.Bräuer A, Weyland W, Fritz U, Schuhmann MU, Schmidt JH, Braun U. Determination of core body temperature. A comparison of esophageal, bladder, and rectal temperature during postoperative rewarming. Anaesthesist. 1997;46(8):683–688. doi: 10.1007/s001010050454. [DOI] [PubMed] [Google Scholar]
- 46.Mac V, Elon L, Mix J, et al. Risk factors for reaching core body temperature thresholds in Florida agricultural workers. J Occup Environ Med. 2021;63(5): 395–402. doi: 10.1097/JOM.0000000000002150. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified data and statistical code will be made available upon reasonable request to the corresponding author.