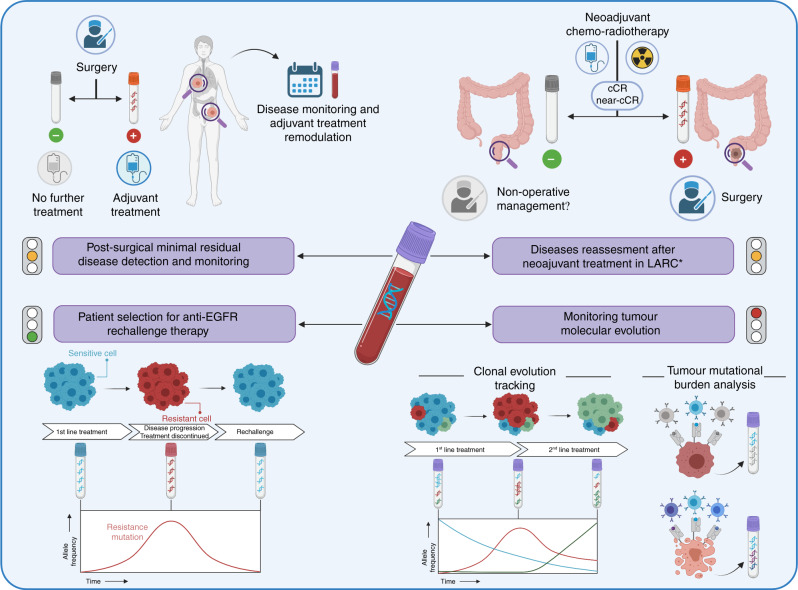
Fig. 1. Interventional liquid biopsy can orient therapeutic decision-making in CRC.
Circulating tumor DNA (ctDNA) analysis through liquid biopsy has proven to be a robust method to tailor personalised treatments for CRC) patient care. Promising results have been achieved in the post-surgical adjuvant setting and in driving treatment choice in locally advanced rectal cancer (LARC) after neoadjuvant treatment. Ongoing and future studies, exploiting ctDNA to guide anti-EGFR rechallenge therapy and treatment choices based on mutational and molecular CRC evaluation, will further expand the use of interventional liquid biopsy in CRC patients care. The “traffic lights”, close to each box where clinical strategies are defined, summarise the level of evidence supporting applicability of interventional ctDNA in the clinical practice. Figure created with BioRender.com. Keys: LARC locally advanced rectal cancer, * ctDNA negativity or positivity might be taken into account in patients treated with neoadjuvant multimodal treatment and achieving near clinical complete response (cCR) or cCR to evaluate if they might be candidate to non-operative management rather than curative surgery, respectively; cCR clinical complete response, EGFR epidermal growth factor receptor, green light = initial prospective data available, orange line = only retrospective data available, red light = only partial data available.