Abstract
Host density shapes infection risk through two opposing phenomena. First, when infective stages are subdivided among multiple hosts, greater host densities decrease infection risk through ‘safety in numbers’. Hosts, however, represent resources for parasites, and greater host availability also fuels parasite reproduction. Hence, host density increases infection risk through ‘density-dependent transmission’. Theory proposes that these phenomena are not disparate outcomes but occur over different timescales. That is, higher host densities may reduce short-term infection risk, but because they support parasite reproduction, may increase long-term risk. We tested this theory in a zooplankton-disease system with laboratory experiments and field observations. Supporting theory, we found that negative density–risk relationships (safety in numbers) sometimes emerged over short timescales, but these relationships reversed to ‘density-dependent transmission’ within two generations. By allowing parasite numerical responses to play out, time can shift the consequences of host density, from reduced immediate risk to amplified future risk.
Keywords: safety in numbers, encounter dilution, density-dependent transmission, timescale, Daphnia, Metschnikowia
1. Background
Individuals within a population can experience reduced risk of consumption when density is high through a phenomenon called ‘safety in numbers’ [1]. For instance, fish often shoal in response to the threat of predation [2]. Because the threat imposed by a predator is shared among the fish in a shoal, a shoal with more individuals inherently possesses lower per capita risk. This phenomenon arises, in part, from predator functional responses, because predator attack rates saturate with increasing prey density due to handling time and satiation [3]. In a simpler sense, this benefit emerges from probabilities: when predator numbers (the numerator) are finite and fixed, and prey numbers (the denominator) increase, individual risk (the quotient) declines [4]. The mathematical basis to safety in numbers implies that it may be extended generally, regardless of the predator or prey under consideration.
Similarities between predator–prey and host–parasite interactions [5–7] suggest that safety in numbers might also extend to hosts when they are exposed to parasites [8]. This can occur when parasite infective stages are removed from the parasite's population via irreversible exposure of hosts (i.e. where a single parasite can only infect one host). Host–parasite systems that replicate the condition of a free-living and finite consumer (parasite) population may be particularly likely to demonstrate safety in numbers [8–10]. For example, many parasites with complex life cycles rely on eggs or other free-living stages for their transmission, and these infective stages tend to be limiting within an environment (their supply depends on the density of the preceding host in the life cycle [11]). Consequently, support for safety in numbers (sometimes termed ‘encounter dilution’ in disease systems) has been reported for interactions between molluscs and trematodes [11,12], fish and ectoparasites [13,14], and reindeer and warble flies [15].
While increasing host density may provide a benefit of reduced individual risk, host density simultaneously benefits the parasite through increased opportunity for transmission [11,16]. This is manifested in mass-action models of parasite transmission [17,18]. Parasite transmission increases as a function of βSZ, where S represents the density of susceptible hosts, Z the density of infective propagules and β the transmission parameter [19,20]. The βSZ product signifies that the more hosts there are in an environment, the greater the probability that a parasite will encounter a host [21,22]. This phenomenon—density-dependent transmission—is fundamental to our understanding of infectious disease [19,23,24] and predicts that host risk should increase with host density via the following steps: increased host density increases the likelihood of a parasite encountering and infecting a host, which then increases parasite reproduction through the numerical response, ultimately yielding greater host risk (the opposite prediction of safety in numbers). When comparing the disparate predictions of safety in numbers and density-dependent transmission, it can be useful to consider the effects of density from the host perspective (how does host risk change with host density?) and the parasite perspective (how does parasite infection success change with host density?). Acknowledging these perspectives then raises a key question: if host density both decreases host risk and increases parasite transmission, then does high host density produce a cost or benefit to the host? In other words, is it safer or riskier to live at high density?
The answer to this question likely depends on the timescale over which it is asked. Increasing timescale can reveal density-dependent processes [25], and theory predicts that safety in numbers will invert to density-dependent transmission over time [26]. While high host density will immediately reduce individual host risk, it should also fuel parasite reproduction (via the numerical response), resulting in greater parasite density and greater host risk in the second parasite generation. These temporal dynamics are supported at evolutionary timescales. Comparative approaches have described greater abundance of parasites in social versus solitary taxa [27,28] and with increasing group size [29,30]. Experimental approaches can augment this evidence by providing mechanistic depictions of how host risk and parasite transmission operate over ecologically relevant timescales [10].
The crustacean Daphnia dentifera and its fungal parasite, Metschnikowia bicuspidata, represent an ideal system for testing theory on density–risk relationships. First, both host and parasite can be maintained in a well-mixed aquatic environment, which mirrors the mass action principles of host–parasite contact rates [31]. Second, the parasite is transmitted during its free-living spore stage, and spore density can be tightly controlled to approximate a fixed and finite parasite population [32–34]. Third, the rapid generation times of both players mean that transmission can be evaluated over multiple generations, and thus over increasing timescales. Finally, the hosts are transparent, such that parasite attack rates and successful attacks can be directly quantified [35]. With these attributes, we evaluated the effects of Daphnia density on host infection risk and Metschnikowia transmission success over two generations of the interaction. Our study combined a laboratory transmission experiment, microscopic evaluations to ground-truth assumptions and field observations of disease dynamics. We found mixed support for safety in numbers, alongside consistent support for density-dependent transmission. Moreover, including increasing timescale revealed that host density could reduce immediate risk of infection for Daphnia, but by supporting Metschnikowia reproduction, also increased Daphnia future risk. Time can therefore reverse density–risk relationships.
2. Methods
(a) . Study system
Our system includes the zooplankton host, Daphnia dentifera and fungal parasite, Metschnikowia bicuspidata. Infection occurs when Daphnia consume fungal spores, which puncture the gut epithelium and enter the body cavity. Inside the body cavity, the fungus undergoes four stages of development and replication [36], ultimately producing thousands of spores that are released upon host death [37]. The within-host life cycle—from initial infection to production of spores—takes approximately ten days under laboratory conditions [36].
(b) . Laboratory transmission experiment
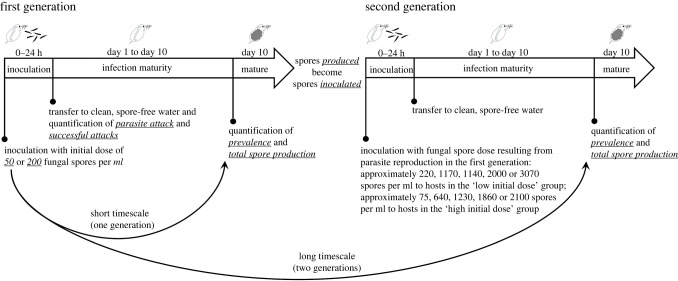
We manipulated host and parasite densities and perpetuated transmission over two generations of the interaction (figure 1). In brief, we began each generation with a starting host density. We exposed hosts in a density treatment to an initial spore dose and measured infection prevalence ten days later. From those hosts, we also quantified how many fungal spores were produced and used spore counts to inoculate the second generation of hosts (while keeping host densities constant). By evaluating the relationship between starting host density and prevalence at the end of the first generation, we captured density–risk relationships on a ‘short’ timescale (over one generation of the interaction). And by evaluating the relationship between starting host density and prevalence at the end of the second generation, we captured density–risk relationships on a ‘long’ timescale (over two generations of the interaction). The transmission experiment used a single clone of Daphnia dentifera (CB 03–15) and a single strain of Metschnikowia bicuspidata. Experimental Daphnia were collected as neonates from mothers maintained in culture and were raised with 1 mg C/L of the alga, Ankistrodesmus falcatus, provided daily. Experimental manipulation began when Daphnia reached seven days of age. Further information regarding Daphnia culture and maintenance is provided in the electronic supplementary materials (S1: Extended Methods).
Figure 1.
Laboratory transmission experiment timeline. We began the experiment by inoculating Daphnia in five host density treatments with a low (50 spores ml−1) or high (200 spores ml−1) initial spore dose. By quantifying prevalence of infection and total spore production ten days later—after one generation of the host–parasite interaction—we evaluated how host density affected host risk and parasite reproduction on a short timescale. Keeping host densities constant, we inoculated hosts in the second generation with spore counts resulting from parasite reproduction in the first generation. By evaluating the relationship between starting host density (i.e. at the beginning of the experiment) and prevalence and parasite reproduction at the end of the second generation, we captured density–risk relationships on a long timescale (over two generations of the interaction). In the first generation, a subset of hosts were assessed microscopically to quantify parasite attacks and the number of successful attacks.
(i) . First generation
Daphnia were allocated to one of five host density treatments: 1, 4, 8, 12 or 16 Daphnia per replicate (electronic supplementary material, table S1). Replicates consisted of 50 ml filtered lake water in tubes, such that host densities were: 0.02, 0.08, 0.16, 0.24 or 0.32 Daphnia ml−1. To start the experiment, each tube received an initial parasite inoculum of either 50 (low dose) or 200 (high dose) spores ml−1, which represent standard spore doses for this system and fall within the range of doses selected for a similar study [38]. The inoculation lasted 24 h, after which hosts were transferred to fresh, spore-free water. Ten days after inoculation, Daphnia were examined with a dissecting microscope for mature infections. Infections were designated as those in which the Daphnia body was full of fungal spores. From each replicate, we also quantified total spore production (the number of fungal spores released by all infected Daphnia). All infected Daphnia from a given replicate were homogenized for two minutes with a grinding implement, and released fungal spores were counted using a haemocytometer under high magnification (compound microscope; 40× objective).
(ii) . Second generation
To begin the second generation of the interaction, new experimental Daphnia (7 days old) were randomly allocated to the same host density treatments. The spore dose provided to each host density treatment in the second generation was equivalent to the average fungal spore production resulting from each host density treatment in the first generation (see figure 1 for a description of the process and figure 2 for spore values). That is, we quantified the average spore production resulting from each host density by initial dose combination from the first generation, then used those averages as the parasite inoculum in the second generation. In that sense, transmission was perpetuated across the two generations. When counting total spore production, two of the 104 tubes that contained infected hosts did not have their spores counted; we imputed values for those two tubes (details available in electronic supplementary materials).
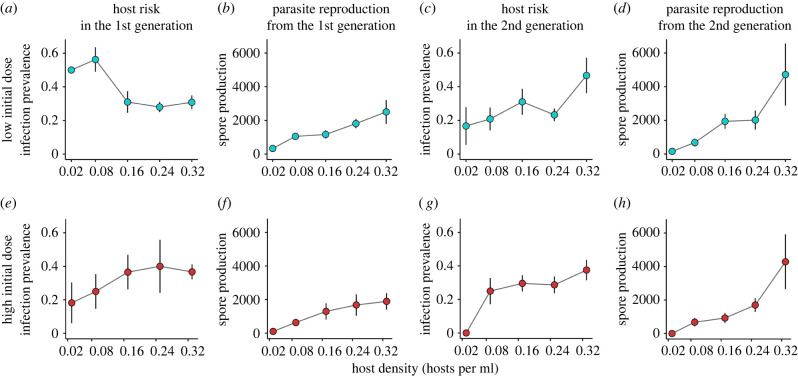
Figure 2.
Effects of host density on host risk and parasite reproduction through time. For each initial spore dose (rows), we provide the relationship between host density and host risk (infection prevalence) and parasite reproduction (spore production) over time. The plots follow the timeline of the experiment (figure 1). (a) In the low initial dose (top row), host risk in the 1st generation declines with density, supporting safety in numbers. (b) However, parasite reproduction resulting from the 1st generation increases with host density. (c) Higher parasite reproduction leads to increased host risk with density in the 2nd generation, supporting density-dependent transmission. (d) The positive relationship between host density and parasite reproduction is maintained at the end of the 2nd generation. Thus, in the low initial dose, we observed safety in numbers reverse to density-dependent transmission with increasing timescale. In the high initial dose, we did not observe safety in numbers. (e) Host risk in the 1st generation trended positively with host density, and (f) parasite reproduction resulting from the 1st generation increased with host density. These same patterns continued in the second generation (g and h). Each point represents the average value from a treatment, with bars for standard error. (Online version in colour.)
(c) . Microscopic evaluations to ground-truth assumptions
Because Daphnia are transparent, we could visualize and evaluate mechanisms involved in density-risk relationships, including how host density shapes parasite attack rates and the success of parasite attacks. We quantified these processes on a subset of individuals from each replicate at the end of the inoculation period (figure 1). Daphnia were examined microscopically, and we counted fungal spores that had partially punctured the host gut epithelium, and spores that had crossed the gut and entered the body cavity. With these spore counts, we quantified two metrics. ‘Attacking spores’ represents the total number of fungal spores that attempted to infect the Daphnia, i.e. the sum of spores partially embedded in the gut and spores that entered the body cavity [35]. ‘Successful attacks’ represents only those fungal spores that successfully infected the body cavity [35]. Attacking spores are those with infection potential, and successful attacks are those with realized infection potential.
(d) . Field observations
We connected previously published field infection data [39] with estimates of Daphnia density to assess density–risk relationships in natural systems. We visited six lakes in Central Indiana over six months to quantify Daphnia density and Metschnikowia prevalence. Every two weeks, each lake was sampled with a 70 µm Wisconsin net. Six vertical tows were conducted during each visit: plankton from the first three tows were pooled as a ‘live’ sample (for infection estimates; available in [39]) and plankton from the second three tows were pooled as a ‘preserved’ sample (for host density estimates). While the previously published study assessed Daphnia prior to Metschnikowia epidemics, the present study focuses on Daphnia collected during epidemics.
(e) . Analyses
We were specifically interested in effects of host density and timescale on infection risk. Because we had no a priori interest regarding how the two standard initial doses would affect infections (and have previously investigated dose effects in [35]), we ran all models independently for both the low initial dose (50 spores ml−1) and high initial dose (200 spores ml−1). This enabled us to focus on effects of interest (host density, and dose variation resulting from the parasite's numerical response). However, understanding the effects of initial dose on probability of infection and other infection patterns remains an important area of disease ecology. For the interested reader, we include results and interpretation of models containing initial dose as a fixed effect (and all potential interactions) in the electronic supplementary materials (Section S2: Full models incorporating initial spore dose).
(i) . The host perspective
With the laboratory transmission experiment, we asked whether host density decreased infection risk (safety in numbers) or increased infection risk (density-dependent transmission), and how the direction of the relationship was affected by increasing timescale. Our metric for host risk was individual infection status (0: uninfected, 1: infected), as this value describes likelihood of infection [11]. We ran a generalized linear mixed model (function ‘glmmTMB’ [40]) with a binomial error distribution testing for effects of host density on infection status. Because individual Daphnia were grouped together in tubes, we incorporated tube ID as a random effect. Our model predictors included host density, generation (first or second) and a host density by generation interaction. We assessed the full model and its nested variants (host density and generation, and host density alone) and compared the fit of each model with AIC (where the lowest AIC indicates the best model fit).
(ii) . The parasite perspective
Switching perspectives, we investigated whether total host density increased parasite reproduction and how this pattern (density-dependent transmission) was affected by increasing timescale. Our metric for parasite reproduction was total spore production. We ran a generalized linear model (function ‘glmmTMB’ [40]) testing for effects of host density on total spore production within a tube. We modelled the residuals with a negative binomial error distribution with zero inflation and included generation as a fixed effect and a host density by generation interaction effect. As above, we used AIC to assess the fit of the full model and its nested variants.
(iii) . Microscopic evaluations
Safety in numbers should occur when parasite infective stages are diluted among an increasing number of potential hosts [8,11,38]. That is, as total host density increases, each host should be exposed to fewer parasites, reducing each individual's probability of infection and, consequently, the population's prevalence. To evaluate whether this condition was met, we assessed how parasite attack rates and successful attacks changed with host density when the number of administered parasites was fixed. Using generalized linear mixed models with negative binomial error distributions, we assessed attacking spores (spores with infection potential) and successful attacks (spores with realized infection potential) as a function of host density. We incorporated tube ID as a random effect because, in some cases, multiple Daphnia were assessed from the same tube. These analyses only contained experimental Daphnia from the first generation (since the second generation received varying spore doses).
We used the results of these models to produce predictions for cumulative parasite attack (how many spores in total attacked hosts?) and cumulative parasite success (how many spores in total successfully infected hosts)? Because our models had evaluated numbers of spores per individual Daphnia (the host perspective), we could extrapolate from these models to calculate the cumulative number of spores that interacted with Daphnia in each density treatment (the parasite perspective). To obtain these values, we used the predict function (Base R [41]) on the four models to obtain model estimates of average number of attacking spores and successful attacks per Daphnia in each host density treatment. We then multiplied per capita estimates by the total number of Daphnia hosts within a density treatment to arrive at cumulative values. In the case that the model had a non-significant effect of host density, we used the predict function on the intercept-only version of the model. We provide a schematic of this approach in the electronic supplementary materials (S3: Predictions for cumulative attack and success).
(iv) . Field observations
We used field observational data to explore density–risk relationships in naturally occurring Daphnia–Metschnikowia interactions and whether these relationships depended on the timescale examined. Using six months of host density and infection prevalence data, we ran generalized linear mixed models with individual infection status as the response variable and log-transformed Daphnia density as the predictor. Because transmission occurs continuously in the field, we denoted individuals as ‘infected’ based on presence of any infection stage (from the earliest ‘spore’ stage, to the final ‘ascus’ stage [36]). Our model structure accounted for temporal autocorrelation and non-independence of lakes (populations of origin) by incorporating a random effect of sampling event nested in lake, with an autoregressive covariance structure (ar1(event + 0 | lake); function ‘glmmTMB’ [40]). We ran the model at three separate timescales: on the immediate relationship between Daphnia density and Metschnikowia prevalence assessed during the same sampling event (t = i); on the lagged relationship between density and prevalence assessed at the subsequent sampling event (t = i + 1; two-week lag); and the lagged relationship between density and prevalence assessed two sampling events later (t = i + 2; four-week lag). The lagged density terms were not correlated with one another (all r < 0.3 and all p > 0.1).
3. Results
(a) . The host perspective
In the low initial dose, the full model containing host density, generation, and a host density by generation interaction fit best (electronic supplementary material, table S1). In this model, host density affected infection status (z = −2.935; p = 0.003), but the direction of the effect depended on the generation of the interaction (host density × generation: z = 3.061; p = 0.002). In the first generation, individual infection risk declined with host density (supporting safety in numbers), but in the second generation, individual infection risk increased with host density (supporting density-dependent transmission; figure 2). Generation was a significant effect in the low initial dose treatment (z = −3.228; p = 0.001). In the high initial dose, the model with host density alone fit best (electronic supplementary material, table S1). Here, host density was marginally positively associated with individual infection risk (z = 1.903; p = 0.057; figure 2). There were no significant effects of generation or the interaction between host density and generation in any of the worse-fitting models. We therefore found evidence for safety in numbers (in the first generation) in the low initial dose, but not in the high initial dose.
(b) . The parasite perspective
We found strong support for density-dependent transmission. In the low initial dose, the model containing host density and generation fit best (electronic supplementary material, table S2). Here, host density increased total spore production (z = 6.129; p < 0.001), with a significant positive effect of generation (z = 2.199; p = 0.028). The host density by generation interaction effect was non-significant (electronic supplementary material, table S2). In the low initial dose, the highest parasite reproduction resulted from the highest host density treatment following two generations of the interaction (figure 2). In the high initial dose, the best-fitting model was that containing host density alone (electronic supplementary material, table S2), where host density increased total spore production (z = 3.89; p < 0.001). There were no significant effects of generation or the interaction between host density and generation (electronic supplementary material, table S2). In the high initial dose, the highest parasite reproduction also resulted from the highest host density treatment following two generations of the interaction (figure 2).
(c) . Microscopic evaluations
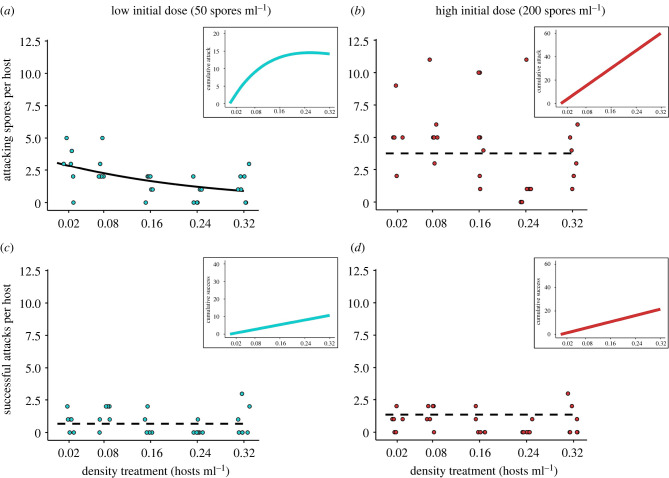
We found limited evidence for safety in numbers when we counted attacking spores and successful attacks on Daphnia. In the low initial dose, the number of attacking spores per Daphnia decreased with host density, supporting safety in numbers (z = −2.910; p = 0.004; figure 3a). In the high initial dose, the relationship between host density and attacking spores was non-significant (z = −1.767; p = 0.077; figure 3b). For successful attacks (spores that entered the body cavity), there was no effect of host density in either low or high initial doses (low: z = −0.792; p = 0.428; high: z = −0.502; p = 0.616; figure 3c,d). Transforming per capita values (the host perspective) to cumulative values (the parasite perspective) indicated that attacking and successful spores had positive responses to host density (figure 3 inset panels). From the host perspective, higher host densities could reduce per capita risk of parasite attacks (but not successful attacks). The parasite perspective, however, demonstrated that higher host densities increased both parasite attack rates and the rates of successful attacks.
Figure 3.
Microscopic evaluations to ground-truth assumptions. Attacking spores per host and successful attacks per host were quantified from a subset of Daphnia after inoculation. (a) In the low initial dose, attacking spores per host decreased with host density, supporting the mechanistic condition for safety in numbers. (b) This relationship was negative but non-significant in the high initial dose. In both doses (c and d), there was no effect of host density on successful attacks per host. Because spores must infect the body cavity to develop into a mature infection, these data do not support a pattern of decreasing host risk with increasing host density. Multiplying per capita averages by the number of hosts in each treatment creates predictions for how parasite cumulative success changes with host density (inset subplots). The cumulative number of spores that attacked a host is positive, saturating with host density in the low initial dose (a); this relationship is linear in the high initial dose (b). The cumulative number of successful attacks also increases linearly with host density (c and d inset subplots). Each point represents an individual Daphnia's count, with lines representing the fit regression (solid: significant, dashed: non-significant). (Online version in colour.)
(d) . Field observations
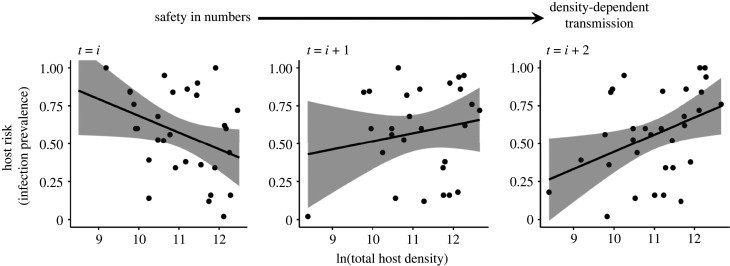
We found field evidence for safety in numbers occurring over immediate timescales and density-dependent transmission occurring over longer timescales. When hosts and parasites were evaluated during the same time period (t = i), there was a marginal negative effect of host density on individual risk of infection (z = −1.936; p = 0.053; figure 4). This relationship flattened out to a null association when individual risk of infection was evaluated as a function of host density two weeks prior (t = i + 1; z = 1.699; p = 0.089; figure 4). When individual infection risk was evaluated as a function of host density four weeks prior (t = i + 2), there was a positive effect of host density on infection prevalence (z = 2.796; p = 0.005). Over the longest timescale, more hosts were associated with higher per capita risk.
Figure 4.
Effects of time on naturally occurring density-risk relationships. Densities of naturally occurring Daphnia in six lakes were evaluated as predictors of Metschnikowia prevalence within the same sampling event (t = i; left), at the subsequent sampling events two weeks later (t = i + 1; centre) and four weeks later (t = i + 2; right). The time lag influences the direction of the relationship. At immediate timescales (t = i), safety in numbers is observed and at increasing timescales (t = i + 2), density-dependent transmission is observed. Shading represents standard error.
4. Discussion
High host densities can have disparate effects on infection, from reduced per capita risk of hosts (safety in numbers) to enhanced transmission of parasites (density-dependent transmission). Theory suggests that the outcome will depend on the timescale over which host–parasite dynamics are observed (i.e. whether the observational window incorporates the parasite's numerical response [26]). We tested this theory and found strong support that Daphnia–Metschnikowia interactions exemplify density-dependent transmission, with positive effects of host density becoming more evident with increasing timescale. In our laboratory transmission experiment, we found evidence for density-dependent transmission, where host density increases parasite reproduction leading to a positive relationship between host density and infection risk. Our microscopic evaluations confirmed that host density can dilute the number of parasites attacking each host (reducing per capita risk from the host's perspective), while simultaneously increasing the parasite's attack rate and rate of successful attacks (enhancing transmission from the parasite's perspective). Finally, field data revealed a clear inversion of density–risk relationships over time: from safety in numbers over short timescales, to density-dependent transmission four weeks later. It has long been suggested that dense groups provide individual-level benefits, because conspecifics can be used as cover from predation or decoys for infection. Our results highlight the rapidity with which parasite numerical responses can erode these benefits.
High host densities can decrease per capita risk, with some caveats. One important factor is the ratio of consumers to resources, or in our case, the ratio of parasites to hosts. When parasites are limiting compared to hosts, hosts may experience declining risk with increasing host density [11]. Conversely, if the density of parasites is sufficiently high, no host will go without risk [26,29,42]. There may then be a threshold parasite-to-host ratio, below which hosts experience safety in numbers and above which density-dependent transmission dominates. Our laboratory transmission experiment supported the importance of parasite–host ratios and appeared to bracket the threshold at which density–risk relationships switch from negative to positive. In the low initial spore dose, the administered exposure levels ranged from 3.125 parasites per ml per host (highest host density treatment) to 50 parasites per ml per host (lowest host density treatment), and we observed a negative density–prevalence relationship. Conversely, our high initial spore dose included exposure levels ranging from 12.5 to 200 parasites per ml per host, and ultimately led to higher rates of successful parasite attack and a general positive relationship between host density and infection prevalence. The parasite-to-host ratio can therefore determine whether or not safety in numbers is observed. Importantly, each experimental Daphnia likely did not ingest all the spores administered to them. Indeed, our microscopic evaluations demonstrated numbers of attacking spores that fell far below the values administered. For example, the treatment with the highest parasite-to-host ratio (1 host exposed to 500 spores per ml) resulted in only five attacking spores per host on average. Daphnia can reduce their foraging rates (an innate defense termed ‘adaptive anorexia’, e.g. [43,44]) shortly following exposure to fungal spores [45]. Indeed, parasite avoidance behaviours are a common strategy to reduce disease among taxa [46,47]. Hosts employing parasite avoidance traits may therefore impose strong limits on per capita exposure levels, with consequences for which parasite-to-host ratios confer safety in numbers benefits.
Consumer success can be encapsulated by the numerical response, or in our study, how parasite density changes with host density. The sequence of events spanning a parasite's initial attack to its release of infective stages takes time and highlights the importance of timescale when relating densities of both players. In measuring parasite reproduction in the laboratory 10 days after host exposure, we observed a positive numerical response: the more hosts that were made available to the parasite, the more parasite offspring were ultimately produced. This numerical response resulted in increasing host risk (prevalence) with host density in the second generation in the laboratory and following a one-month time lag in the field. Collectively, these results emphasize the scale-dependence of density–risk relationships and clarify discrepancies between past Daphnia studies. For instance, safety in numbers was observed in Daphnia following a single experimental generation [38], while longer-term studies found strong evidence of density-dependent transmission [22]. Our results indicate that these findings are not contradictory, but merely capture separate time periods of the interaction.
Safety in numbers can best operate if detection of a group by a consumer, as well as the consumer's attack rate, do not increase proportionally with prey/host density [4,8,9,13,15]. Hence, consumer traits create key criteria mediating the direction of density–risk relationships. While a large group of prey can serve as an attractant for predators—increasing probability of detection—we can ignore this aspect in our disease system because infective spores are not actively searching for hosts [37]. However, it is important to consider how the parasite's attack rate, or the per-parasite probability of attacking a host, increases with host density. Predator attack rates often conform to nonlinear functional responses, saturating with increasing prey density due to handling time and satiation [3]. Our system, however, deviates from predator–prey systems in two important ways. First, fungal spores encounter hosts at random, which can allow mass action to shape host–parasite encounter rates [48]. Second, each spore can only attack and infect one host, removing consideration of satiation and handling time. These traits suggest that a single spore's rate of attacking a host should increase linearly with host density. Our microscopic evaluations, however, did not entirely fit this linear approximation. In the low initial dose, we observed reduced attack rates with increasing host density (this relationship was marginal in the high initial dose), resulting in a positive, saturating relationship between host density and cumulative parasite attacks. This result may be explained by increased host competition in high-density treatments, which has been shown to result in suppressed Daphnia foraging and, subsequently, reduced per capita exposure [38,45]. A host's risk of attack may then be shaped by more than just the host density denominator and may be subject to indirect effects arising from competition [38]. Our understanding of how parasite attacks scale with host density may benefit from a dual consideration of parasite traits that initiate attack, and host traits that increase or decrease contact with attacking parasites.
While the parasite functional response dictates the attack rate, the host dose–response curve may be equally important in determining whether a given attack is successful. Host–parasite interactions follow a classic dose-dependent relationship, with greater exposure to attacking parasites resulting in higher probability of host infection [49]. When dividing infective stages among a group of hosts, the host dose–response curve will then determine whether the per capita level of attack is sufficient to result in infection. These curves suggest that parasite attacks could become unsuccessful at the highest host densities (where per capita attack rates occupy the lower end of the dose–response curve; [50]) and therefore indicate that host susceptibility can shape the degree to which successful transmission versus safety in numbers scales with host density [51]. While the dilution of infective stages with increasing host density has been met with support in diverse systems [13,41,52], we did not observe this pattern. This may be due to Daphnia gut barrier defenses that can block attacking parasites and decouple the relationship between attack and successful infection [35]. In line with this, we observed no differences in per capita rates of successful attacks across host densities: Daphnia in the highest host density treatments had the same number of spores entering their body cavities as those in the lowest density treatments. These constant levels of parasite success imply that Daphnia in all treatments shared an equal probability of developing mature infections, thereby removing the importance of the dose–response curve for density–risk relationships (while Daphnia clones can vary in their ability to clear early infections, our study used only one clone to standardize these clearance probabilities). Switching from the host perspective to the parasite perspective illustrated an important outcome of constant parasite success among hosts. We observed a positive, linear relationship between host density and the cumulative number of successfully attacking spores, emphasizing that parasite success can be a strict function of host supply.
The field brings with it many real-world complexities not seen in the laboratory—host densities do not stay constant, population genetic structure can shift, and other ecological players can influence transmission. Despite these complexities, we observed a strong signal for safety in numbers inverting to density-dependent transmission in the field. This finding highlights that host densities can immediately reduce per capita risk but can also promote risk in the long term. Simulations have described these same inversions, demonstrating that any beneficial effects of host density can rapidly decay as transmission is allowed to occur [50]. This outcome is similarly predicted by theoretical consumer–resource models (including those for predator–prey interactions [26]). The difference between host–parasite and predator–prey systems is that the inversion is expected to occur more quickly for parasites because of their rapid generation times. That this inversion occurs has applications that extend beyond theory. For instance, whether farmed oysters amplify or dilute pathogens of wild oysters depends on the time that farmed oysters are allowed to remain in a system before harvest—i.e. whether there is sufficient time for the parasite numerical response to promote transmission [51]. Both increasing host density [14] and culling [53] have been suggested as management strategies that can reduce disease. Our results indicate that elevating host density will only reduce disease if the added hosts act as true dead-ends for transmission. Adding hosts with even a slight ability to transmit will ultimately produce parasite infective stages, rather than absorbing them [51]. Given the rapidity with which safety in numbers reverses to density-dependent transmission, ecologists and managers alike should exercise caution with expectations that host density will decrease disease risk.
5. Conclusion
We empirically assessed how time modifies infection risk through its effects on host and parasite densities and found that, because time allows for transmission, high host density can result in high host risk in as little as two generations of parasite reproduction. Our results highlight that addressing density–risk relationships requires a side-by-side comparison of the host and parasite perspectives, with the latter allowing us to evaluate the capacity of host populations to promote parasite reproduction and transmission. In our study, we simplified some responses with linear models; nevertheless, nonlinear approaches may be powerful for addressing theory in the future. Both the saturating response of parasite attack rates with host density, and the potential for dose–response curves to reduce parasite success at the highest host densities, may result in unimodal relationships between density and infection, fitting with theory that transmission is a nonlinear process [20,24]. Models and experiments containing broad and ecologically relevant host and parasite densities will be critical for understanding when group living benefits or harms hosts.
Acknowledgements
Thank you to Shalyn Kaiser, Ilona Menel, Dianna Oleksyn, and Anna Osborne, all of whom provided assistance with data collection.
Data accessibility
Some of the data supporting the results of this study are already publicly available on Dryad Digital Repository: https://doi.org/10.5061/dryad.v15dv41ts [54]. Additional data and supporting R scripts are avaialable at https://doi.org/10.5061/dryad.g4f4qrft2 [55].
Electronic supplementary material is available online [56].
Authors' contributions
T.E.S.M.: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, visualization, writing—original draft, writing—review and editing; C.E.C.: funding acquisition, methodology, resources, supervision, writing—review and editing; S.G.: data curation, investigation, writing—review and editing; V.R.L.: data curation, investigation, writing—review and editing; Z.T.S.: data curation, investigation, writing—review and editing; J.C.B.: conceptualization, formal analysis, funding acquisition, methodology, project administration, supervision, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
This research was supported by the National Science Foundation (NSF 1420273 [awarded to J.C.B.], DGE 1144245 [awarded to T.E.S.M.]; NSF 1354407 [awarded to C.E.C.]; and NSF 1701515 [awarded to C.E.C. and T.E.S.M.]). Any opinion, findings and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation.
References
- 1.Alexander RD. 1974. The evolution of social behavior. Annu. Rev. Ecol. Syst. 5, 325-383. ( 10.1146/annurev.es.05.110174.001545) [DOI] [Google Scholar]
- 2.Godin J-GJ. 1986. Antipredator function of shoaling in teleost fishes: a selective review. Le Naturaliste canadien 113, 241-250. [Google Scholar]
- 3.Holling CS. 1959. Some characteristics of simple types of predation and parasitism. Can. Entomol. 91, 385-398. ( 10.4039/Ent91385-7) [DOI] [Google Scholar]
- 4.Lehtonen J, Jaatinen K. 2016. Safety in numbers: the dilution effect and other drivers of group life in the face of danger. Behav. Ecol. Sociobiol. 70, 449-458. ( 10.1007/s00265-016-2075-5) [DOI] [Google Scholar]
- 5.Raffel TR, Martin LB, Rohr JR. 2008. Parasites as predators: unifying natural enemy ecology. Trends Ecol. Evol. 23, 610-618. ( 10.1016/j.tree.2008.06.015) [DOI] [PubMed] [Google Scholar]
- 6.Hall SR, et al. 2008. Is infectious disease just another type of predator-prey interaction? In Infectious disease ecology: the effects of the ecosystem on disease and of disease on ecosystems. Princeton, NJ: Princeton University Press. [Google Scholar]
- 7.Lafferty KD, DeLeo G, Briggs CJ, Dobson AP, Gross T, Kuris AM. 2015. A general consumer-resource population model. Science 349, 854-857. ( 10.1126/science.aaa6224) [DOI] [PubMed] [Google Scholar]
- 8.Mooring MS, Hart BL. 1992. Animal grouping for protection from parasites: selfish herd and encounter-dilution effects. Behaviour 123, 173-193. ( 10.1163/156853992X00011) [DOI] [Google Scholar]
- 9.Hart BL. 1994. Behavioural defense against parasites: interaction with parasite invasiveness. Parasitology 109, S139-S151. ( 10.1017/S0031182000085140) [DOI] [PubMed] [Google Scholar]
- 10.Côté IM, Poulin R. 1995. Parasitism and group size in social animals: a meta-analysis. Behav. Ecol. 6, 159-165. ( 10.1093/beheco/6.2.159) [DOI] [Google Scholar]
- 11.Buck JC, Hechinger RF, Wood AC, Stewart TE, Kuris AM, Lafferty KD. 2017. Host density increases parasite recruitment but decreases host risk in a snail–trematode system. Ecology 98, 2029-2038. ( 10.1002/ecy.1905) [DOI] [PubMed] [Google Scholar]
- 12.Magalhães L, Freitas R, Dairain A, De Montaudouin X. 2017. Can host density attenuate parasitism? J. Mar. Biol. Assoc. UK 97, 497-505. ( 10.1017/S0025315416001107) [DOI] [Google Scholar]
- 13.Poulin R, FitzGerald GJ. 1989. Shoaling as an anti-ectoparasite mechanism in juvenile sticklebacks (Gasterosteus spp.) Behav. Ecol. Sociobiol. 24, 251-255. ( 10.1007/BF00295205) [DOI] [Google Scholar]
- 14.Samsing F, Oppedal F, Johansson D, Bui S, Dempster T. 2014. High host densities dilute sea lice Lepeophtheirus salmonis loads on individual Atlantic salmon, but do not reduce lice infection success. Aquacult. Env. Interac. 6, 81-89. ( 10.3354/aei00118) [DOI] [Google Scholar]
- 15.Fauchald P, Rødven R, Bårdsen B-J, Langeland K, Tveraa T, Yoccoz NG, Ims RA. 2007. Escaping parasitism in the selfish herd: age, size and density-dependent warble fly infestation in reindeer. Oikos 116, 491-499. ( 10.1111/j.0030-1299.2007.15390.x) [DOI] [Google Scholar]
- 16.Buck JC, Lutterschmidt WI. 2017. Parasite abundance decreases with host density: evidence of the encounter-dilution effect for a parasite with a complex life cycle. Hydrobiologia 784, 201-210. ( 10.1007/s10750-016-2874-8) [DOI] [Google Scholar]
- 17.Anderson RM, May RM. 1979. Population biology of infectious diseases: Part I. Nature 280, 361-367. ( 10.1038/280361a0) [DOI] [PubMed] [Google Scholar]
- 18.Anderson RM, May RM. 1981. The population dynamics of microparasites and their invertebrate hosts. Phil. Trans. R. Soc. B 291, 451-524. ( 10.1098/RSTB.1981.0005) [DOI] [Google Scholar]
- 19.Antonovics J. 2017. Transmission dynamics: critical questions and challenges. Phil. Trans. R Soc. B 372, 20160087. ( 10.1098/rstb.2016.0087) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McCallum H, et al. 2017. Breaking beta: deconstructing the parasite transmission function. Phil. Trans. R. Soc. B 372, 20160084. ( 10.1098/rstb.2016.0084) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tien HJ, Earn DJD. 2010. Multiple transmission pathways and disease dynamics in a waterborne pathogen model. B. Math. Biol. 72, 1506-1533. ( 10.1007/s11538-010-9507-6) [DOI] [PubMed] [Google Scholar]
- 22.Dallas TA, Krkosek M, Drake JM. 2017. Experimental evidence of a pathogen invasion threshold. R. Soc. Open Sci. 5, 171975. ( 10.1098/rsos.171975) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McCallum H, Barlow N, Hone J. 2001. How should pathogen transmission be modelled? Trends Ecol. Evol. 16, 295-300. ( 10.1016/S0169-5347(01)02144-9) [DOI] [PubMed] [Google Scholar]
- 24.Hopkins SR, Fleming-Davies AE, Belden LK, Wojdak JM. 2020. Systematic review of modelling assumptions and empirical evidence: Does parasite transmission increase nonlinearly with host density? Methods Ecol. Evol. 11, 476-486. ( 10.1111/2041-210X.13361) [DOI] [Google Scholar]
- 25.Hassell MP, Latto J, May RM. 1989. Seeing the wood for the trees: detecting density-dependence from existing life-table studies. J. Anim. Ecol. 58, 883-892. ( 10.2307/5130) [DOI] [Google Scholar]
- 26.Buck JC, Lafferty KD. Efficiency, patchiness, and scale interact to alter density-dependent risk in a consumer-resource model. In revision. [Google Scholar]
- 27.Arneberg P, Skorping A, Grenfell B, Read AF. 1998. Host densities as determinants of abundance in parasite communities. Proc. R. Soc. Lond. B 265, 1283-1289. ( 10.1098/rspb.1998.0431) [DOI] [Google Scholar]
- 28.Arneberg P. 2001. An ecological law and its macroecological consequences as revealed by studies of relationships between host densities and parasite prevalence. Ecography 24, 352-358. ( 10.1034/j.1600-0587.2001.240313.x) [DOI] [Google Scholar]
- 29.Rifkin JL, Nunn CL, Garamszegi LZ. 2012. Do animals living in larger groups experience greater parasitism? A meta-analysis. Am. Nat. 180, 70-82. ( 10.1086/666081) [DOI] [PubMed] [Google Scholar]
- 30.Patterson JEH, Ruckstuhl KE. 2013. Parasite infection and host group size: a meta-analytical review. Parasitology 140, 803-813. ( 10.1017/S0031182012002259) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Regoes RR, Hottinger JW, Sygnarski L, Ebert D. 2003. The infection rate of Daphnia magna by Pasteuria ramosa conforms with the mass-action principle. Epidemiol. Infect. 131, 957-966. ( 10.1017/S0950268803008793) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cáceres CE, Knight CJ, Hall SR. 2009. Predator-spreaders: predation can enhance parasite success in a planktonic host–parasite system. Ecology 90, 2850-2858. ( 10.1890/08-2154.1) [DOI] [PubMed] [Google Scholar]
- 33.Hite JL, Penczykowski RM, Shocket MS, Strauss AT, Orlando PA, Duffy MA, Cáceres CE, Hall SR. 2016. Parasites destabilize host populations by shifting stage-structured interactions. Ecology 97, 439-449. ( 10.1890/15-1065.1) [DOI] [PubMed] [Google Scholar]
- 34.Strauss AT, Bowling AM, Duffy MA, Cáceres CE, Hall SR. 2018. Linking host traits, interactions with competitors and disease: mechanistic foundations for disease dilution. Funct. Ecol. 32, 1271-1279. ( 10.1111/1365-2435.13066) [DOI] [Google Scholar]
- 35.Stewart Merrill TE, Hall SR, Merrill L, Cáceres CE. 2019. Variation in immune defense shapes disease outcomes in laboratory and wild Daphnia. Int. Comp. Biol. 59, 1203-1219. ( 10.1093/icb/icz079) [DOI] [PubMed] [Google Scholar]
- 36.Stewart Merrill TE, Cáceres CE. 2018. Within-host complexity of a plankton-parasite interaction. Ecology 99, 2864-2867. ( 10.1002/ecy.2483) [DOI] [PubMed] [Google Scholar]
- 37.Ebert D. 2005. Ecology, epidemiology, and evolution of parasitism in daphnia. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information. See https://www.ncbi.nlm.nih.gov/books/NBK2036/?term=Ecology%2C%20epidemiology%2C%20and%20evolution%20of%20parasitism%20in%20daphnia. [Google Scholar]
- 38.Civitello D, Pearsall S, Duffy MA, Hall SR. 2013. Parasite consumption and host interference can inhibit disease spread in dense populations. Ecol. Lett. 16, 626-634. ( 10.1111/ele.12089) [DOI] [PubMed] [Google Scholar]
- 39.Stewart Merrill, TE, Hall SR, Cáceres CE. 2021. Data from: Parasite exposure and host susceptibility jointly drive the emergence of epidemics. Dryad Digital Respository. ( 10.5061/dryad.v15dv41ts) [DOI] [PubMed]
- 40.Brooks ME, Kristensen K, van Benthem KJ, Magnusson A, Berg CW, Nielsen A, Skaug HJ, Maechler M, Bolker BM. 2017. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R. J. 9, 378-400. ( 10.32614/RJ-2017-066) [DOI] [Google Scholar]
- 41.R Core Team. 2021. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. See https://www.R-project.org/. [Google Scholar]
- 42.Song Z, Proctor H. 2020. Parasite prevalence in intermediate hosts increases with waterbody age and abundance of final hosts. Oecologia 192, 311-321. ( 10.1007/s00442-020-04600-4) [DOI] [PubMed] [Google Scholar]
- 43.Hite JL, Cressler CE. 2019. Parasite-mediated anorexia and nutrition modulate virulence evolution. Integr. Comp. Biol. 59, 1264-1274. ( 10.1093/icb/icz100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hite JL, Pfenning AC, Cressler CE. 2020. Starving the enemy? Feeding behavior shapes host-parasite interactions. Trends Ecol. Evol. 35, 68-80. ( 10.1016/j.tree.2019.08.004) [DOI] [PubMed] [Google Scholar]
- 45.Strauss AT, Hite JL, Civitello DJ, Shocket MS, Cáceres CE, Hall SR. 2019. Genotypic variation in parasite avoidance behaviour and other mechanistic, nonlinear components of transmission. Proc. R. Soc. B. 286, 20192164. ( 10.1098/rspb.2019.2164) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Buck JC, Weinstein SB, Young HS. 2018. Ecological and evolutionary consequences of parasite avoidance. Trends Ecol. Evol. 33, 619-632. ( 10.1016/j.tree.2018.05.001) [DOI] [PubMed] [Google Scholar]
- 47.Weinstein SB, Buck JC, Young HS. 2018. A landscape of disgust. Science 359, 1213-1214. ( 10.1126/science.aas8694) [DOI] [PubMed] [Google Scholar]
- 48.Rapti Z, Stewart Merrill TE, Mueller-Brennan L, Kavouras JH, Cáceres CE. 2019. Indirect effects in a planktonic disease system. Theor. Popul. Biol. 130, 132-142. ( 10.1016/j.tpb.2019.07.009) [DOI] [PubMed] [Google Scholar]
- 49.Stewart Merrill TE, Hall SR, Cáceres CE. 2021. Parasite exposure and host susceptibility jointly drive the emergence of epidemics. Ecology 102, e03245. ( 10.1002/ecy.3245) [DOI] [PubMed] [Google Scholar]
- 50.Bidegain G, Powell EN, Klinck JM, Hofmann EE, Ben-Horin T, Bushek D, Ford SE, Munroe DM, Guo X. 2017. Modeling the transmission of Perkinsus marinus in the Eastern oyster Crassostrea virginica. Fish. Res. 186, 82-93. ( 10.1016/j.fishres.2016.08.006) [DOI] [Google Scholar]
- 51.Ben-Horin T, Burge CA, Bushek D, Groner ML, Proestou DA, Huey LI, Bidegain G, Carnegie RB. 2018. Intensive oyster aquaculture can reduce disease impacts on sympatric wild oysters. Aquacult. Env. Interac. 10, 557-567. ( 10.3354/aei00290) [DOI] [Google Scholar]
- 52.Reeson AF, Wilson K, Cory JS, Hankard P, Weeks JM, Goulson D, Hails RS. 2000. Effects of phenotypic plasticity on pathogen transmission in the field in a Lepidoptera-NPV system. Oecologia 124, 373-380. ( 10.1007/s004420000397) [DOI] [PubMed] [Google Scholar]
- 53.Henriksen EH, Frainer A, Knudsen R, Kristoffersen R, Kuris AM, Lafferty KD, Amundsen P-A. 2019. Fish culling reduces tapeworm burden in Arctic charr by increasing parasite mortality rather than by reducing density-dependent transmission. J. Appl. Ecol. 56, 1482-1491. ( 10.1111/1365-2664.13369) [DOI] [Google Scholar]
- 54.Stewart Merrill TE, Cáceres CE, Gray S, Laird VR, Schnitzler ZT, Buck JC. 2022. Parasite exposure and host susceptibility jointly drive the emergence of epidemics. Dryad Digital Repository. ( 10.5061/dryad.v15dv41ts) [DOI] [PubMed]
- 55.Stewart Merrill TE, Cáceres CE, Gray S, Laird VR, Schnitzler ZT, Buck JC. 2022. Data from: Timescale reverses the relationship between host density and infection risk. Dryad Digital Repository. ( 10.5061/dryad.g4f4qrft2) [DOI] [PMC free article] [PubMed]
- 56.Stewart Merrill TE, Cáceres CE, Gray S, Laird VR, Schnitzler ZT, Buck JC. 2022. Timescale reverses the relationship between host density and infection risk. Figshare. ( 10.6084/m9.figshare.c.6115282) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Stewart Merrill, TE, Hall SR, Cáceres CE. 2021. Data from: Parasite exposure and host susceptibility jointly drive the emergence of epidemics. Dryad Digital Respository. ( 10.5061/dryad.v15dv41ts) [DOI] [PubMed]
- Stewart Merrill TE, Cáceres CE, Gray S, Laird VR, Schnitzler ZT, Buck JC. 2022. Parasite exposure and host susceptibility jointly drive the emergence of epidemics. Dryad Digital Repository. ( 10.5061/dryad.v15dv41ts) [DOI] [PubMed]
- Stewart Merrill TE, Cáceres CE, Gray S, Laird VR, Schnitzler ZT, Buck JC. 2022. Data from: Timescale reverses the relationship between host density and infection risk. Dryad Digital Repository. ( 10.5061/dryad.g4f4qrft2) [DOI] [PMC free article] [PubMed]
- Stewart Merrill TE, Cáceres CE, Gray S, Laird VR, Schnitzler ZT, Buck JC. 2022. Timescale reverses the relationship between host density and infection risk. Figshare. ( 10.6084/m9.figshare.c.6115282) [DOI] [PMC free article] [PubMed]
Data Availability Statement
Some of the data supporting the results of this study are already publicly available on Dryad Digital Repository: https://doi.org/10.5061/dryad.v15dv41ts [54]. Additional data and supporting R scripts are avaialable at https://doi.org/10.5061/dryad.g4f4qrft2 [55].
Electronic supplementary material is available online [56].