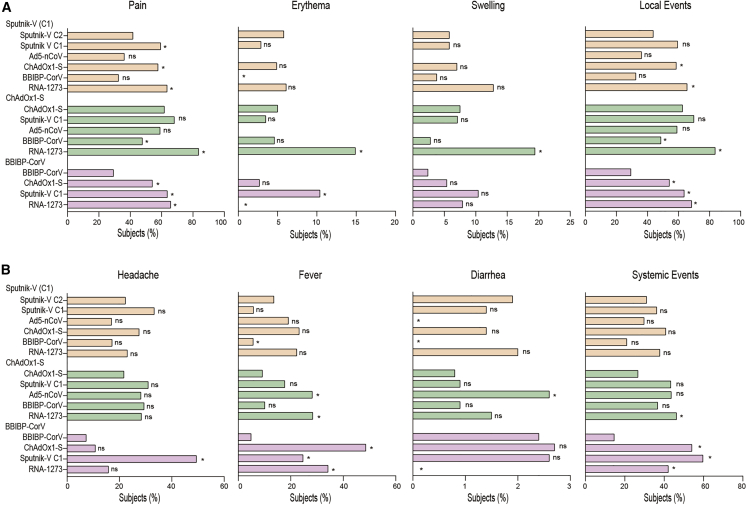
Figure 2.
Adverse events of solicited local and systemic reactions in days 0–7 following the application of the second vaccine dose by study arm via telephone contacting
Adverse events included either local reactions (A) (pain, erythema, and swelling) or systemic reactions (B) (headache, fever, and diarrhea). The proportion of participants with local or systemic adverse event was reported by vaccine schedule, and statistical analysis was performed using the χ2 test. Statistical significance is shown with the following notations: ∗p < 0.05, ns, not significant. Sputnik V C1 vaccine (rAd26, Gamaleya), Sputnik V C2 vaccine (rAd5, Gamaleya), ChAdOx1-S vaccine (AstraZeneca), BBIBP-CorV vaccine (Sinopharm), Ad5-nCoV vaccine (CanSino), mRNA-1273 vaccine (Moderna).