Key Points
Question
What is the effect of a graded sensorimotor retraining intervention on pain intensity for adults with chronic low back pain?
Findings
In this randomized clinical trial that included 276 participants, a graded sensorimotor retraining intervention, compared with a sham procedure and attention control, resulted in a statistically significant improvement in pain intensity at 18 weeks (estimated mean difference, 1.0 point on an 11-point numeric rating scale [range, 0-10 points]).
Meaning
Among patients with chronic low back pain, a graded sensorimotor retraining intervention, compared with a sham procedure and attention control, significantly improved pain intensity at 18 weeks, although the improvements were modest and further research in other patient populations is needed to understand the generalizability of the findings.
Abstract
Importance
The effects of altered neural processing, defined as altering neural networks responsible for perceptions of pain and function, on chronic pain remains unclear.
Objective
To estimate the effect of a graded sensorimotor retraining intervention (RESOLVE) on pain intensity in people with chronic low back pain.
Design, Setting, and Participants
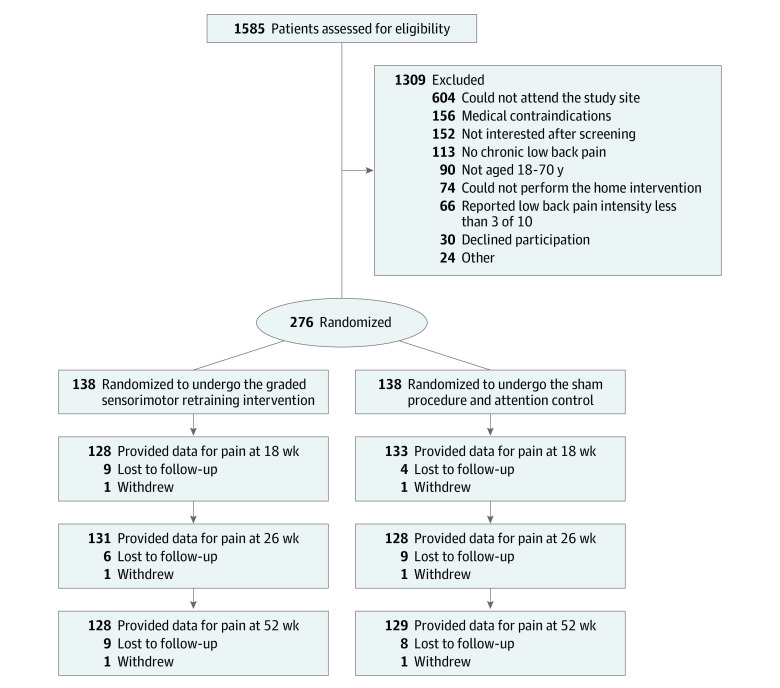
This parallel, 2-group, randomized clinical trial recruited participants with chronic (>3 months) nonspecific low back pain from primary care and community settings. A total of 276 adults were randomized (in a 1:1 ratio) to the intervention or sham procedure and attention control groups delivered by clinicians at a medical research institute in Sydney, Australia. The first participant was randomized on December 10, 2015, and the last was randomized on July 25, 2019. Follow-up was completed on February 3, 2020.
Interventions
Participants randomized to the intervention group (n = 138) were asked to participate in 12 weekly clinical sessions and home training designed to educate them about and assist them with movement and physical activity while experiencing lower back pain. Participants randomized to the control group (n = 138) were asked to participate in 12 weekly clinical sessions and home training that required similar time as the intervention but did not focus on education, movement, and physical activity. The control group included sham laser and shortwave diathermy applied to the back and sham noninvasive brain stimulation.
Main Outcomes and Measures
The primary outcome was pain intensity at 18 weeks, measured on an 11-point numerical rating scale (range, 0 [no pain] to 10 [worst pain imaginable]) for which the between-group minimum clinically important difference is 1.0 point.
Results
Among 276 randomized patients (mean [SD] age, 46 [14.3] years; 138 [50%] women), 261 (95%) completed follow-up at 18 weeks. The mean pain intensity was 5.6 at baseline and 3.1 at 18 weeks in the intervention group and 5.8 at baseline and 4.0 at 18 weeks in the control group, with an estimated between-group mean difference at 18 weeks of −1.0 point ([95% CI, −1.5 to −0.4]; P = .001), favoring the intervention group.
Conclusions and Relevance
In this randomized clinical trial conducted at a single center among patients with chronic low back pain, graded sensorimotor retraining, compared with a sham procedure and attention control, significantly improved pain intensity at 18 weeks. The improvements in pain intensity were small, and further research is needed to understand the generalizability of the findings.
Trial Registration
ANZCTR Identifier: ACTRN12615000610538
This randomized clinical trial examines the effects of graded sensorimotor retraining, compared with an attention control with sham procedure, in patients with moderate to severe chronic low back pain.
Introduction
In 2019, low back pain affected approximately 52 million people in the US and 568 million people worldwide.1,2 Based on data from 1990 to 2019, low back pain was the leading cause of years lived with disability worldwide.1 A meta-analysis from 2013 indicated that one-third of people with low back pain experienced persistent pain and disability 3 months after symptom onset,3 and these individuals were unlikely to recover completely within 1 year.3
New effective treatments are needed for low back pain.4,5,6 Graded sensorimotor retraining (RESOLVE) is a novel intervention designed to alter how people think about their body in pain, how they process sensory information from their back, and how they move their back during activities.
This randomized clinical trial evaluated the effects of graded sensorimotor retraining, compared with an attention control with sham procedure, in people with moderate to severe chronic low back pain.
Methods
Study Design and Participants
This parallel, 2-group, randomized clinical trial was approved by the University of New South Wales Human Research Ethics Committee (HC15357). The trial protocol is shown in Supplement 1. Protocols published during the trial7,8 did not include secondary outcomes that were process variables, and these outcomes are not reported here. The secondary outcome of Insomnia Severity Index was omitted from the published protocol7 in error. Participants were recruited between November 1, 2015, and July 28, 2019. The first participant was randomized on December 10, 2015, and follow-up was completed on February 3, 2020. All participants gave written informed consent.
Participants were recruited in Sydney, Australia, by direct referral from primary care clinicians as well as by advertisements in the community using local and online media. People were eligible for inclusion if they reported back pain with a pain intensity rating of at least 3 of 10 on an 11-point numerical rating scale that persisted for at least 12 consecutive weeks, regardless of whether they had associated leg pain. Additional inclusion criteria were age between 18 and 70 years, fluency in the English language, ability to access the internet, and availability of a trusted person to assist with the home portion of the intervention. People with radicular pain, with low back pain caused by a serious medical condition (ie, infection, fracture, malignancy), who were pregnant or gave birth in the past 6 months, who had undergone spinal surgery in the past 12 months or were scheduled for major surgery in the next 12 months, with an uncontrolled mental health condition that would impede participation, and with contraindications to physical activity, transcranial direct current stimulation, cranial electrical stimulation, low-intensity laser therapy, or short-wave diathermy were excluded.
Randomization and Masking
Eligible participants were randomly allocated (in a 1:1 ratio) to receive the intervention or matched attention control with sham procedures. Participants were informed that both group interventions contained treatments that targeted central nervous system function, combined with treatments directed toward functioning of the back. Participants were informed that some of the treatments were not active. No further information was disclosed publicly to maintain the integrity of blinding. Participants were not required to stop ongoing treatment for their low back pain.
A randomization sequence was generated using computer-generated block randomization (random block size range, 2-10). Randomizations were sealed in opaque, sequentially numbered envelopes. Eligible participants were randomly assigned immediately after baseline assessment by opening the next numbered envelope, at which point they were considered enrolled. The trial clinicians were unblinded, but participants, researchers collecting outcome data, and researchers analyzing outcome data were masked to group identity.
Procedures
Graded Sensorimotor Retraining
The graded sensorimotor retraining intervention was delivered by a physiotherapist (n = 6) or an exercise physiologist (n = 1). The intervention consisted of 12 individual treatment sessions lasting up to 1 hour scheduled over 12 to 18 weeks. The intervention included a home treatment component that participants were encouraged to complete for 30 minutes 5 times per week during the treatment period, recording their participation in an intervention diary. The primary intention was to help people in pain understand that it was safe and helpful to move (step 1), feel safe to move (step 2), and experience that it was safe to move (step 3) as they progressed toward reengagement with meaningful functional goals. The first session included contemporary pain education delivered using graphical media, video, metaphor, and narrative and continued for the duration of the intervention (step 1). In sessions 2 through 12, graded premovement treatments were provided in the clinic and continued at home, which involved sensory precision training and mental rehearsal of movement (step 2). In the sixth session, participants began graded movement training in the clinic and continued at home, starting with simple spinal movements before progressing toward more complex exercises, such as squatting, lunging, and lifting, which continued for the duration of the intervention. Each treatment session followed a standardized protocol with mandatory progression (eFigure in Supplement 2). The full intervention description is provided in the eMethods in Supplement 2.
Attention Control With Sham Procedures
The attention control with sham procedures was delivered by a physiotherapist (n = 3), 2 of whom also delivered the graded sensorimotor retraining intervention. Participants randomized to the control group underwent 12 1-hour sessions over a period of 12 to 18 weeks. Sessions took place approximately weekly. The control group was designed to control for time with an expert clinician and the graded progression of treatment in the intervention.
The first 2 sessions included discussion of the participant’s low back pain experience without advice or education provided. Sessions 3 through 12 included sham low-intensity laser therapy to the most painful area of the back and sham transcranial direct current stimulation applied to the motor and prefrontal cortices on the side contralateral to their worst pain.9 In the sixth session, participants began sham shortwave diathermy over the back, which continued for the duration of the intervention. To match the intervention’s home training component, participants were provided with a sham cranial electrical stimulation device and instructed to use it at home for 30 minutes 5 times a week and record their use in a diary. Treatment adherence to the intervention and control was assessed by recording the attendance of participants at each treatment session.
Data Collection
Data were collected at baseline (prior to randomization) and at 18, 26, and 52 weeks after randomization using mailed questionnaires and secure online data-capture software. Demographic and clinical characteristics (age, sex, work status, educational level, duration of current back pain episode, number of previous episodes, self-reported use of prescribed and over-the-counter medicines for low back pain, other areas of pain, and back pain–related compensation status) were obtained at the baseline assessment prior to randomization, along with all primary and secondary outcome measurements. No data were collected on race, ethnicity, or ancestry.
Outcome Measurements
Primary Outcome
The primary outcome was mean pain intensity over the previous week assessed using an 11-point numerical rating scale10 (range, 0 [no pain] to 10 [worst pain imaginable]) at 18 weeks after randomization. The between-group minimal clinically important difference (MCID) is 1.0 point on the 11-point numerical rating scale.11 The within-group MCID is a 30% change from baseline.12
Secondary Outcomes
Secondary outcomes were disability, assessed using the 24-item Roland-Morris Disability Questionnaire13 (scale range, 0-24; higher scores indicate greater disability; within-group MCID, 30% from baseline)12; health-related quality of life, assessed using the 5-level EuroQoL-5 dimensions14 questionnaire (EQ-5D-5L; scale range, 1-5; higher scores indicate better quality of life; within-group MCID, 0.03 from baseline15) and the EuroQol-5D health thermometer(range, 0-100; higher scores indicate better quality of life; within-group MCID, 10.5)15; depressive symptoms, assessed using the depression subscale of the Depression Anxiety Stress Scale (range, 0-21; higher scores indicate worse outcome; MCID not established); sleep quality (insomnia), assessed using the Insomnia Severity Index (range 0-28; higher scores indicate worse insomnia; MCID not established); beliefs about low back pain, assessed using the Back Beliefs Questionnaire (range 9-45; lower scores indicate more pessimistic beliefs about the consequences of low back pain; MCID not established); kinesiophobia, assessed using the Tampa Scale of Kinesiophobia (range, 17-68; higher scores are worse; MCID not established); catastrophizing, assessed using the Pain Catastrophizing Scale (range, 0-52; higher scores are worse; MCID not established); the Pain Self-Efficacy Questionnaire (range, 0-60; higher scores are better; within-group MCID, 5.5); and treatment rationale credibility, assessed using the Credibility and Expectancy Questionnaire16 (scale range, 0-48; lower scores are worse, MCID not established).
Serious adverse events and adverse events were reported by the patient and recorded by the trial therapist at the beginning of each treatment session.
Additional Prespecified Outcomes
Additional prespecified outcomes consist of process variables (2-point discrimination distance, left-right judgement task performance [accuracy and speed], Neurophysiology of Pain Questionnaire score, Fremantle Back Awareness Questionnaire score, Movement Imagery Questionnaire-Revised score, and Elgueta-Cancino Pelvic Tilt Test) and cost-effectiveness, which will be reported separately.
Post Hoc Outcomes
Post hoc outcomes consisted of recovery, defined as pain intensity in the past week rated less than or equal to 1 on the 11-point numerical rating scale at both 18 and 26 weeks after randomization (MCID not established); response to intervention, defined as at least 30% reduction in pain intensity from baseline to 18 weeks; response to intervention, defined as at least 50% reduction in pain intensity from baseline to 18 weeks; and global perceived effect, assessed using the Global Back Recovery Scale (range, −5 to 5; lower scores indicate poorer recovery; MCID not established).
Sample Size Calculation
The trial was designed to detect a mean (SD) between-group difference of 1.0 (2.0) point in pain intensity at the primary end point of 18 weeks. A 1.0-point difference on an 11-point numerical rating scale has been suggested as the threshold for clinical importance for a between-group difference (between-group MCID) in clinical trials investigating interventions for chronic pain.11 Sample size was calculated for an interaction effect between time (4 repeated observations) and group randomization, using an estimated interobservation correlation of base 0.6 with decay rate 0.1, significance level of .05, and power of 80%.17 With a 1:1 treatment randomization and anticipating a 15% loss to follow-up, the trial aimed to recruit 276 participants. After 15 participants had been randomized, an error was detected in the specification of repeated measures used for the sample size calculation. A recalculation required the sample to be increased from 266 to 276 and for disability, measured by the Roland Morris Disability Questionnaire, to be changed from a primary outcome to a secondary outcome to maintain statistical power for a single primary outcome—pain intensity.
Statistical Analysis
Mean values are presented for normally distributed data and median values with IQRs are presented for skewed data. The mean between-group difference on the primary outcome of pain intensity and the secondary outcomes of disability, quality of life, depressive symptoms, insomnia, back beliefs, kinesiophobia, pain catastrophizing, pain self-efficacy, and global perceived effect were estimated with linear mixed models that incorporated group randomization, time, and the treatment × time interaction term. Time was modeled as a 4-level categorical variable, which assumed observations were collected at the stipulated time. The model was robust to data missing at random and accounted for baseline outcome values. For continuous outcomes, the estimated effects were the differences in mean values between the groups at 18 weeks, 26 weeks, and 52 weeks and the overall difference across all time points. For the post hoc dichotomous outcomes, recovery and response, the percentage of participants in either group was compared using the Fisher exact test. The effect of the intervention was expressed as the absolute difference in risk, and the ratio of risks, between groups. Credibility was compared between groups using an independent samples t test. The full analysis set included all randomized participants who provided data at each time point analyzed. For all statistical analyses, participant data were analyzed in the groups to which they were randomized, regardless of adherence. Missing data were considered trivial for the primary outcome of pain intensity at 18 weeks, and assumed to be missing at random. Therefore, multiple imputation was not performed.
All effects were estimated with a 95% CI and all statistical tests were 2-sided with α level of .05. Because of the potential for type I error due to multiple comparisons, findings for analyses of secondary outcomes should be interpreted as exploratory.
The mean effect of the intervention on pain intensity at 18 weeks on the principal stratum of participants who would adhere to the randomized intervention regardless of which intervention group they were randomized to (the complier mean causal effect) was estimated using instrumental variable regression with allocation as the instrumental variable.18 The complier mean causal effect analysis was specified in the statistical analysis plan.8 For this analysis, (observed) adherence to the (randomized) intervention was defined as attending at least 9 treatment sessions, ie, 75% of the intervention. Sensitivity analyses were conducted to examine the robustness of conclusions to different assumptions in the complier mean causal effect population (the principal compliers). The statistical analyses were completed using R, version 3.3.3 (R Foundation), or Stata, version 10 (StataCorp), and validated independently by a second investigator.
Results
A total of 276 participants were randomized. Figure 1 shows the flow of participants through the trial. The trial had sufficient funding to obtain follow-up observations for all participants at 18 weeks. At this time, a single additional observation was collected from participants who had not yet completed follow-up, after which the trial was closed because additional funding was unavailable. Thirty-four participants were contacted early to provide the 26-week follow-up and 45 were contacted early to provide the 52-week follow-up data. Overall, 261 participants (95%) provided data for the primary outcome at 18 weeks, 259 (94%) at 26 weeks, and 257 (93%) at 52 weeks. For the 9 secondary outcomes, the median (range) numbers of missing data values were 29 (19-29) at 18 weeks, 30 (23-31) at 26 weeks, and 40 (30-42) at 52 weeks. The median (IQR) time to follow-up at each time point was 19.3 (18-20) weeks at week 18, 27.6 (26-27.7) weeks at week 26, and 48.5 (47.6-53.6) weeks at week 52.
Figure 1. Flow of Participants in a Study of the Effect of Sensorimotor Retraining on Pain Intensity in Chronic Low Back Pain.
Table 1 shows baseline characteristics of participants. The mean (SD) age was 46 (14.3) years and 138 of 276 participants (50%) were women. Participants reported symptoms of back pain for a median (IQR) of 5 (3.0-8.1) years. At baseline, pain intensity based on the numerical rating scale pain intensity (score range, 0-10) was moderate to high (mean [SD] score, 5.7 [1.8]), disability (score range, 0-24; 0 indicates the best outcome) was moderate (mean [SD] score, 9.8 [5.2]), health-related quality of life (score range, 0-100; 100 indicates the best outcome) was moderate (mean [SD] score, 63.7 [19.1]), depressive symptoms (score range, 0-21; 0 indicates the best outcome) were low (mean [SD] score, 4.8 [5.0]), and sleep problems (score range, 0-28; 0 indicates the best outcome) were moderate (mean [SD] score, 13.7 [7.6]). Twenty-four percent of participants were taking opioid analgesics and 79% had recently consulted health care professionals for low back pain. The median (IQR) number of sessions attended was 12 (12-12). In total, 119 participants (86.2%) attended at least 9 sessions of the test intervention and 121 (87.7%) attended at least 9 sessions of the control.
Table 1. Baseline Characteristics in a Study of the Effect of Sensorimotor Retraining on Pain Intensity in Chronic Low Back Pain.
| Characteristic | No. (%) | |
|---|---|---|
| Intervention (n = 138) | Control (n = 138) | |
| Age, mean (SD), y | 44.7 (14.5) | 47.0 (14.1) |
| Women | 72 (52) | 66 (48) |
| Men | 66 (48) | 72 (52) |
| Duration of current episode of LBP, median (IQR), y | 5 (4-10) | 6 (3-11) |
| No. | 137 | 132 |
| Other areas of pain | ||
| Shoulder | 32 (23) | 39 (28) |
| Hip | 32 (23) | 41 (30) |
| Knee | 28 (20) | 30 (22) |
| Leg | 26 (19) | 31 (22) |
| Neck | 13 (9) | 13 (9) |
| Arm | 11 (8) | 12 (9) |
| Feet | 9 (7) | 8 (6) |
| Other | 8 (6) | 10 (7) |
| Work absence or reduction | 27 (20) | 26 (19) |
| Compensation claimed | 9 (7) | 17 (12) |
| Highest education level | (n = 137) | |
| Bachelor’s degree or higher | 77 (56) | 77 (56) |
| Diploma | 19 (14) | 20 (15) |
| Vocational certificate | 17 (12) | 18 (13) |
| High school | ||
| Year 12 | 18 (13) | 10 (7) |
| Year 10 | 7 (5) | 12 (9) |
| Depressive symptom score, mean (SD)a | 4.6 (5.0) | 5.0 (4.9) |
| Insomnia Severity Index, mean (SD)b | 13.5 (7.7) | 14.0 (7.5) |
| Prescription medicine use | ||
| Strong opioidsc | 24 (17) | 30 (22) |
| Weak opioidsc | 5 (4) | 16 (12) |
| Anticonvulsants | 11 (8) | 9 (7) |
| Antidepressants | 4 (3) | 10 (7) |
| Benzodiazepines | 6 (4) | 6 (4) |
| Nonsteroidal anti-inflammatory drugs | 30 (22) | 29 (21) |
| Over-the-counter medicine use | ||
| Acetaminophen (paracetamol) | 64 (46) | 62 (45) |
| Nonsteroidal anti-inflammatory drugs | 55 (40) | 65 (47) |
| Health care use in past 30 d | ||
| General practitioner (family physician) | 55 (40) | 57 (41) |
| Other (eg, allied health professional, alternative medicine) | 70 (51) | 60 (43) |
Depression subscale from the Depression Anxiety and Stress Scale (range, 0-21; higher scores indicate more depressive symptoms; minimal clinically important difference [MCID] not established).
Insomnia Severity Index (range, 0-28; higher scores indicate worse sleep quality; MCID not established).
Opioids were classified by receptor-binding affinity (strong or weak), as generally indicated in the literature (eg, in the study by Chou et al19).
Primary Outcome
Between baseline and 18-week follow-up, mean pain intensity decreased from 5.6 to 3.1 in the intervention group and from 5.8 to 4.0 in the control group (estimated mean difference, −1.0 [95% CI, −1.5 to −0.4]; P = .001) (Table 2 and Figure 2).
Table 2. Estimates of Clinical Efficacy for Primary and Secondary Outcomes in a Study of the Effect of Sensorimotor Retraining on Pain Intensity in Chronic Low Back Pain.
| Outcome | Intervention | Control | Treatment effect, mean (95% CI)a | P value | ||
|---|---|---|---|---|---|---|
| Mean (SD) | No. of participants | Mean (SD) | No. of participants | |||
| Primary outcome (18-wk value) | ||||||
| Pain intensityb | ||||||
| Baseline | 5.6 (1.8) | 138 | 5.8 (1.8) | 138 | ||
| 18 wk | 3.1 (2.4) | 128 | 4.0 (2.5) | 133 | −1.0 (−1.5 to −0.4) | .001 |
| 26 wk | 3.3 (2.4) | 131 | 3.9 (2.4) | 128 | −0.7 (−1.2 to −0.1) | .02 |
| 52 wk | 3.5 (2.7) | 128 | 4.0 (2.7) | 129 | −0.5 (−1.1 to 0.1) | .09 |
| Overall intervention effectc | −0.6 (−1.0 to 0.1) | .01 | ||||
| Secondary outcomes | ||||||
| Disabilityd | ||||||
| Baseline | 9.6 (5.4) | 138 | 10 (5.0) | 138 | ||
| 18 wk | 3.6 (4.6) | 127 | 6.4 (5.8) | 130 | −2.6 (−3.9 to −1.3) | <.001 |
| 26 wk | 4.0 (5.0) | 126 | 6.3 (5.8) | 127 | −2.1 (−3.4 to −0.8) | .002 |
| 52 wk | 4.1 (5.4) | 122 | 6.1 (5.8) | 124 | −1.8 (−3.1 to −0.5) | .008 |
| Overall intervention effect | −1.7 (−2.8 to −0.6) | .003 | ||||
| Quality of life (EQ-5D-5L)e | ||||||
| Baseline | 0.6 (0.3) | 138 | 0.5 (0.3) | 138 | ||
| 18 wk | 0.8 (0.3) | 122 | 0.7 (0.3) | 125 | 0.1 (0.0 to 0.1) | .02 |
| 26 wk | 0.7 (0.3) | 120 | 0.7 (0.3) | 125 | 0.1 (0.0 to 0.1) | .05 |
| 52 wk | 0.7 (0.3) | 114 | 0.7 (0.3) | 120 | 0.1 (0.0 to 0.1) | .05 |
| Overall intervention effect | 0.1 (0.0 to 0.1) | .06 | ||||
| Quality of life (0-100)f | ||||||
| Baseline | 62.0 (20) | 138 | 65.3 (18) | 138 | ||
| 18 wk | 78.3 (16.8) | 122 | 73.1 (17.3) | 125 | 4.6 (0.1 to 9.1) | .05 |
| 26 wk | 77.1 (17.4) | 120 | 71.8 (18.1) | 125 | 4.4 (−0.1 to 9.0) | .06 |
| 52 wk | 76.3 (18.9) | 114 | 70.5 (20.7) | 121 | 5.0 (0.4 to 9.6) | .03 |
| Overall intervention effect | 2.7 (−1.1 to 6.4) | .16 | ||||
| Depressive symptom scoreg | ||||||
| Baseline | 4.6 (5.0) | 138 | 5 (4.9) | 138 | ||
| 18 wk | 2.4 (4.0) | 122 | 3.2 (4.6) | 125 | −0.8 (−2.0 to 0.3) | .16 |
| 26 wk | 2.4 (4.2) | 120 | 3.5 (4.8) | 126 | −1.0 (−2.1 to 0.2) | .10 |
| 52 wk | 2.8 (4.6) | 114 | 3.2 (4.6) | 122 | −0.3 (−1.5 to 0.9) | .63 |
| Overall intervention effect | −0.6 (−1.6 to 0.4) | .22 | ||||
| Insomnia Severity Index (0-28)h | ||||||
| Baseline | 13.5 (7.7) | 138 | 14.0 (7.5) | 138 | ||
| 18 wk | 8.4 (7.4) | 122 | 10.3 (7.7) | 125 | −1.8 (−3.7 to 0.1) | .06 |
| 26 wk | 8.9 (7.5) | 120 | 10.8 (7.7) | 126 | −1.5 (−3.3 to 0.4) | .12 |
| 52 wk | 9.0 (7.6) | 114 | 10.5 (7.7) | 121 | −1.4 (−3.2 to 0.5) | .16 |
| Overall intervention effect | −1.3 (−2.9 to 0.3) | .12 | ||||
| Back beliefsi | ||||||
| Baseline | 26.2 (6.6) | 138 | 25.6 (6.4) | 138 | ||
| 18 wk | 34.7 (6.9) | 122 | 29.2 (6.8) | 126 | 5.4 (3.6 to 7.1) | <.001 |
| 26 wk | 33.5 (7.1) | 120 | 29.5 (6.9) | 126 | 3.8 (2.1 to 4.3) | <.001 |
| 52 wk | 33.8 (7.2) | 114 | 29.6 (7.5) | 122 | 3.8 (2.1 to 5.5) | <.001 |
| Overall intervention effect | 3.4 (2.0 to 4.8) | <.001 | ||||
| Kinesiophobiaj | ||||||
| Baseline | 38.5 (7.6) | 138 | 38.4 (7.3) | 138 | ||
| 18 wk | 29.1 (7.8) | 122 | 35.4 (8.3) | 126 | −6.3 (−8.2 to −4.3) | <.001 |
| 26 wk | 29.5 (8.1) | 120 | 35.3 (7.5) | 126 | −5.4 (−7.3 to −3.4) | <.001 |
| 52 wk | 29.4 (8.2) | 113 | 35.2 (8.2) | 122 | −5.2 (−7.2 to −3.2) | <.001 |
| Overall intervention effect | −4.2 (−5.8 to −2.6) | <.001 | ||||
| Pain catastrophizingk | ||||||
| Baseline | 19.1 (12.7) | 138 | 21.0 (12.8) | 138 | ||
| 18 wk | 8.1 (9.6) | 122 | 15.2 (12.8) | 126 | −6.8 (−9.7 to −3.8) | <.001 |
| 26 wk | 8.1 (10.5) | 120 | 14.2 (12.5) | 126 | −5.7 (−8.6 to −2.7) | <.001 |
| 52 wk | 9.7 (11.6) | 114 | 14.5 (12.7) | 122 | −4.4 (−7.4 to −1.4) | .004 |
| Overall intervention effect | −4.7 (−7.3 to −2.1) | <.001 | ||||
| Pain self-efficacyl | ||||||
| Baseline | 40.4 (13.7) | 138 | 38.7 (13.0) | 138 | ||
| 18 wk | 51.0 (11.1) | 122 | 44.5 (12.7) | 125 | 6.1 (2.9 to 9.2) | <.001 |
| 26 wk | 50.3 (11.9) | 120 | 44.5 (13.3) | 126 | 5.0 (1.8 to 8.1) | .002 |
| 52 wk | 50.5 (12.8) | 114 | 45.0 (13.1) | 122 | 4.6 (1.4 to 7.7) | .005 |
| Overall intervention effect | 4.3 (1.6 to 7.1) | .002 | ||||
The mean treatment effect is from a linear mixed effects model that implicitly controls for the baseline value of the outcome.
Based on an 11-point numerical pain scale (range, 0-10; higher scores indicate more severe pain; between-group minimum clinically important difference [MCID], 1.0 point; within-group MCID, 30% change from baseline).
P value is from a mixed effects model, comparing between-group differences over the entire 52-week trial.
Based on the Roland-Morris Disability Questionnaire (scale range, 0-24; higher scores indicate more disability; within-group MCID, 30% change from baseline).
Based on the 5-level EuroQoL-5 dimensions questionnaire (scale range, 1-5; higher scores indicate better self-rated quality of life; within-group MCID, 0.03 from baseline).
Based on the EuroQol-5D health thermometer (scale range, 0-100; higher scores indicate a better health state; within-group MCID, 10.5 points from baseline).
Based on the depression subscale of the Depression Anxiety and Stress Scale (scale range, 0-21; higher scores indicate more depressive symptoms; MCID not established).
Based on the Insomnia Severity Index (scale range, 0-28; higher scores indicate worse sleep quality; MCID not established).
Based on the Back Beliefs Questionnaire (scale range, 9-45; lower scores indicate more pessimistic beliefs about the consequences of low back pain; MCID not established).
Based on the Tampa Scale of Kinesiophobia (scale range, 17-68; higher scores indicate more severe kinesiophobia; MCID not established).
Based on the Pain Catastrophizing Scale (scale range, 0-52; higher scores indicate more severe catastrophizing, MCID not established).
Based on the Pain Self-Efficacy Questionnaire (scale range, 0-60; higher scores indicate greater self-efficacy; within-group MCID, 5.5 points from baseline).
Figure 2. Outcomes in a Study of the Effect of Sensorimotor Retraining on Pain Intensity in Chronic Low Back Pain.
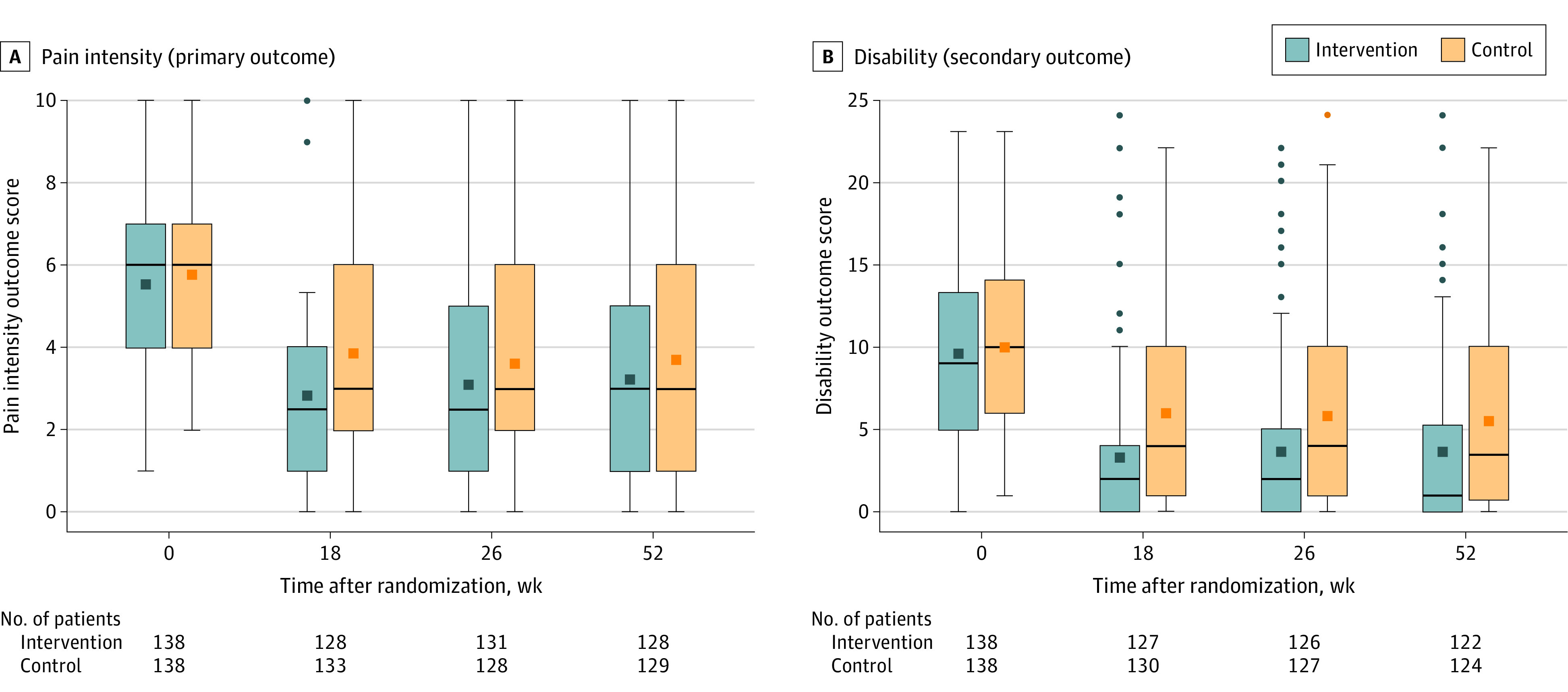
Data presented are observed (ie, as measured) pain and disability scores during the trial period (the time from baseline to 18, 26, and 52 weeks after randomization) for the full analysis set. The middle lines within each box represent the median observed score, the squares in the boxes represent the mean observed score, the box tops and bottoms represent the IQR, the whiskers extend to the most extreme observed values within 1.5 times the IQR of the nearer quartile, and the squares beyond these points represent the observed values outside that range. More negative values indicate lower pain intensity and disability. A, The primary outcome pain intensity score using an 11-point numeric rating scale ranging from 0 (no pain) to 10 (worst pain imaginable); minimal clinically important difference, 1 point. B, The secondary outcome disability score using the Roland Morris Disability Questionnaire ranging from 0 (no disability) to 24 (high disability); minimal clinically important difference, 30% from baseline.
Secondary Outcomes
Compared with participants in the control group over 18 weeks, participants in the intervention group had significantly improved disability (−2.6 points [95% CI, −3.9 to −1.3]; P < .001), quality of life dimensions questionnaire (0.1 points [95% CI, 0.0-0.1] P = .02), health thermometer (4.6 points [95% CI, 0.1-9.1]; P = .05), back beliefs (5.4 points [95% CI, 3.6-7.1]; P < .001), kinesiophobia (−6.3 points [95% CI, −8.2 to −4.3]; P < .001), pain catastrophizing (−6.8 points [95% CI, −9.7 to −3.8]; P < .001), and pain self-efficacy (6.1 points [95% CI, 2.9-9.2]; P < .001) scores. There was no significant effect of the intervention on depressive symptoms (−0.8 points [95% CI, −2.0 to 0.3]; P = .16) or insomnia (−1.8 points [95% CI, −3.7 to 0.1]; P = .06). Results for pain intensity and secondary outcomes at 26 weeks and 52 weeks are shown in Table 2.
At 2 weeks after randomization, there was no statistically significant difference between the 2 groups in the credibility of the interventions and participants’ treatment expectations: mean of 29.3 in the intervention group vs 27.4 in the control group (between-group difference, 1.9 points [95% CI, −0.3 to 4.2]; P = .10).
Post Hoc Outcomes
At the 26-week follow-up, 24 of 131 participants (18.3%) in the intervention group and 13 of 133 (9.8%) in the control group met the criterion for recovery (Fisher exact test P = .04; risk difference, 10% [95% CI, 0.0%-20%]; risk ratio, 1.9 [95% CI, 1.0-3.5]). At the 18-week follow-up, 87 participants (68.0%) in the intervention group and 66 (49.6%) in the control group had a 30% or greater decrease in pain intensity from baseline (risk difference, 20% [95% CI, 10%-30%]; P = .002; risk ratio, 1.4 [95% CI, 1.1-1.7]; P = .003). At the 18-week follow-up, 54 participants (42.2%) in the intervention group and 40 (30%) in the control group had a 50% or greater decrease in pain intensity (risk difference, 10% [95% CI, 0%-30%]; P = .04; risk ratio, 1.4 [95% CI, 1.0-1.9]; P = .04).
At the 18-week follow-up, the global perceived effect changed from 0.2 to 2.6 in the intervention group and from 0 to 1.6 in the control group (between-group difference, 1.1 points [95% CI, 0.6-1.5], P < .001). Estimates for 26 weeks and 52 weeks are listed in Table 3.
Table 3. Estimates of Clinical Efficacy for Post Hoc Outcomes in a Study of the Effect of Sensorimotor Retraining on Pain Intensity in Chronic Low Back Pain.
| Outcome | Intervention | Control | Treatment effect (95% CI) | ||
|---|---|---|---|---|---|
| Mean (SD) | No. of participants | Mean (SD) | No. of participants | ||
| Global perceived effect scorea,b | |||||
| Baseline | 0.2 (1.3) | 137 | 0 (1.3) | 135 | |
| 18 wk | 2.6 (1.7) | 122 | 1.6 (1.9) | 125 | 1.1 (0.6 to 1.5) |
| 26 wk | 2.2 (2.0) | 120 | 1.6 (1.9) | 126 | 0.6 (0.2 to 1.1) |
| 52 wk | 2.4 (2.1) | 114 | 1.6 (2.3) | 121 | 0.7 (0.3 to 1.2) |
| Overall intervention effect, No./total No. (%) | 0.7 (0.3 to 1.0) | ||||
| Recovery (pain intensity ≤1) at 26 wkc | 24/131 (18.3) | 13/133 (9.8) | 10% (0% to 20%) | ||
| Reduction in pain intensity at 18 wk | |||||
| 30% | 87/128 (68.0) | 66/133 (49.6) | 20% (10% to 30%) | ||
| 50% | 54/128 (42.2) | 40/133 (30) | 10% (0% to 30%) | ||
The mean treatment effect is from a linear mixed effects model that implicitly controls for the baseline value of the outcome.
Based on the Global Back Recovery Scale (scale range, −5 to 5; lower scores indicate poorer recovery; minimal clinically important difference not established).
Recovery was defined as pain intensity in the past week rated ≤1 on the 11-point numerical rating scale at both 18 and 26 weeks after randomization (minimal clinically important difference not established).
The complier mean causal effect of the intervention on pain intensity at 18 weeks was −1.1 points (95% CI, −1.7 to −0.4) on the numeric rating scale (range, 0-10).
Adverse Events
No serious adverse events were reported. Two adverse events, both transient general musculoskeletal pain, were reported in the control group. One adverse event, short-term exacerbation of existing low back pain, was reported in the intervention group. All 3 adverse events were considered related to study participation.
Discussion
In this randomized clinical trial of patients with chronic low back pain, graded sensorimotor retraining significantly improved pain intensity at 18 weeks by −1.0 point on the numeric rating scale ranging from 0 to 10, compared with a sham procedure and attention control. This improvement in pain intensity was modest, but it was consistent with a clinically meaningful between-group difference.11
A 30% change from baseline is suggested as a threshold for minimal clinically important within-group change in pain intensity.12 At 18 weeks, 20% more participants met this threshold in the intervention group than in the control group. Of 9 secondary outcomes, 7 (78%) significantly improved in the intervention group, compared with the control. However, because between-group MCID values have not been established for these 7 outcomes, the clinical relevance of these effects is unclear.
Some previous clinical trials of commonly used nonpharmacological interventions for chronic low back pain, such as physical therapy interventions (manual therapy and exercise therapy), were at risk of bias due to low methodological quality.20 Sham controls of nonpharmacological interventions for chronic low back pain, as used in this clinical trial, can reduce bias but are uncommon.21 In recent systematic reviews, pooled effects of exercise therapy (2 trials: mean difference −0.8 on the 11-point pain intensity numerical rating scale)22 and manual therapy (8 trials: mean difference −0.8 on the 11-point pain intensity numerical rating scale)23 compared with sham showed, similar benefits on pain intensity as the effect estimates reported here.
The advantages of the intervention studied here include that rates of adverse events were low. Other interventions for low back pain, such as medications, surgery, and spinal cord stimulation, have a greater number of adverse effects. For example, opioids are associated with physical dependence, hyperalgesia, and life-threatening respiratory depression at high doses.24 Other medicines recommended for low back pain, including nonsteroidal anti-inflammatory drugs, have adverse effects such as gastrointestinal or cardiovascular symptoms. Guidelines for the management of low back pain caution against pharmacological intervention for these reasons.5 Future studies should compare the intervention studied here with pharmacological treatments.
Limitations
This study has several limitations. First, treating clinicians were not masked to group randomization. Second, insufficient resources were available to collect data at 26-week and at 52-week follow-ups in all participants. Third, outcome measures were all self-reported. This may have exaggerated differences between the intervention and control groups for participants who were aware of their group assignment. Fourth, the study was conducted at a single center, raising questions about generalizability of results, including in different racial and ethnic groups. Fifth, the analytic plan assumed that data were missing at random, but it is possible that data were not missing at random.
Conclusions
In this randomized clinical trial conducted at a single center among patients with chronic low back pain, graded sensorimotor retraining, compared with a sham procedure and attention control, significantly improved pain intensity at 18 weeks. The improvements in pain intensity were small, and further research is needed to understand the generalizability of the findings.
Trial protocol
eMethods. Content of the Interventions (TIDieR checklist)
eFigure 1. Timeline for intervention delivery
eReferences
Data sharing statement
References
- 1.Abbafati C, Machado DB, Cislaghi B, et al. ; GBD 2019 Diseases and Injuries Collaborators . Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204-1222. doi: 10.1016/S0140-6736(20)30925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD results. Global Data Health Exchange . Accessed March 19, 2022. https://ghdx.healthdata.org/gbd-results-tool
- 3.da C Menezes Costa L, Maher CG, Hancock MJ, McAuley JH, Herbert RD, Costa LO. The prognosis of acute and persistent low-back pain: a meta-analysis. CMAJ. 2012;184(11):e613-e624. doi: 10.1503/cmaj.111271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hartvigsen J, Hancock MJ, Kongsted A, et al. ; Lancet Low Back Pain Series Working Group . What low back pain is and why we need to pay attention. Lancet. 2018;391(10137):2356-2367. doi: 10.1016/S0140-6736(18)30480-X [DOI] [PubMed] [Google Scholar]
- 5.Foster NE, Anema JR, Cherkin D, et al. ; Lancet Low Back Pain Series Working Group . Prevention and treatment of low back pain: evidence, challenges, and promising directions. Lancet. 2018;391(10137):2368-2383. doi: 10.1016/S0140-6736(18)30489-6 [DOI] [PubMed] [Google Scholar]
- 6.Buchbinder R, van Tulder M, Öberg B, et al. ; Lancet Low Back Pain Series Working Group . Low back pain: a call for action. Lancet. 2018;391(10137):2384-2388. doi: 10.1016/S0140-6736(18)30488-4 [DOI] [PubMed] [Google Scholar]
- 7.Bagg MK, Hübscher M, Rabey M, et al. The RESOLVE trial for people with chronic low back pain: protocol for a randomised clinical trial. J Physiother. 2017;63(1):47-48. doi: 10.1016/j.jphys.2016.11.001 [DOI] [PubMed] [Google Scholar]
- 8.Bagg MK, Lo S, Cashin AG, et al. The RESOLVE trial for people with chronic low back pain: statistical analysis plan. Braz J Phys Ther. 2021;25(1):103-111. doi: 10.1016/j.bjpt.2020.06.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double-blind sham-controlled clinical studies in brain stimulation. Clin Neurophysiol. 2006;117(4):845-850. doi: 10.1016/j.clinph.2005.12.003 [DOI] [PubMed] [Google Scholar]
- 10.Dworkin RH, Turk DC, Farrar JT, et al. ; IMMPACT . Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113(1-2):9-19. doi: 10.1016/j.pain.2004.09.012 [DOI] [PubMed] [Google Scholar]
- 11.Busse JW, Bartlett SJ, Dougados M, et al. Optimal strategies for reporting pain in clinical trials and systematic reviews: recommendations from an OMERACT 12 workshop. J Rheumatol. 2015;42(10):1962-1970. doi: 10.3899/jrheum.141440 [DOI] [PubMed] [Google Scholar]
- 12.Ostelo RWJG, Deyo RA, Stratford P, et al. Interpreting change scores for pain and functional status in low back pain: towards international consensus regarding minimal important change. Spine (Phila Pa 1976). 2008;33(1):90-94. doi: 10.1097/BRS.0b013e31815e3a10 [DOI] [PubMed] [Google Scholar]
- 13.Roland M, Morris R. A study of the natural history of back pain: part I: development of a reliable and sensitive measure of disability in low-back pain. Spine (Phila Pa 1976). 1983;8(2):141-144. doi: 10.1097/00007632-198303000-00004 [DOI] [PubMed] [Google Scholar]
- 14.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20(10):1727-1736. doi: 10.1007/s11136-011-9903-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soer R, Reneman MF, Speijer BLGN, Coppes MH, Vroomen PCAJ. Clinimetric properties of the EuroQol-5D in patients with chronic low back pain. Spine J. 2012;12(11):1035-1039. doi: 10.1016/j.spinee.2012.10.030 [DOI] [PubMed] [Google Scholar]
- 16.Devilly GJ, Borkovec TD. Psychometric properties of the credibility/expectancy questionnaire. J Behav Ther Exp Psychiatry. 2000;31(2):73-86. doi: 10.1016/S0005-7916(00)00012-4 [DOI] [PubMed] [Google Scholar]
- 17.Kreidler SM, Muller KE, Grunwald GK, et al. GLIMMPSE: online power computation for linear models with and without a baseline covariate. J Stat Softw. 2013;54(10):i10. doi: 10.18637/jss.v054.i10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dunn G, Maracy M, Tomenson B. Estimating treatment effects from randomized clinical trials with noncompliance and loss to follow-up: the role of instrumental variable methods. Stat Methods Med Res. 2005;14(4):369-395. doi: 10.1191/0962280205sm403oa [DOI] [PubMed] [Google Scholar]
- 19.Chou R, Deyo R, Friedly J, et al. Systemic pharmacologic therapies for low back pain: a systematic review for an American College of Physicians clinical practice guideline. Ann Intern Med. 2017;166(7):480-492. doi: 10.7326/M16-2458 [DOI] [PubMed] [Google Scholar]
- 20.Cashin AG, Lee H, Bagg MK, et al. A systematic review highlights the need to improve the quality and applicability of trials of physical therapy interventions for low back pain. J Clin Epidemiol. 2020;126(0):106-115. doi: 10.1016/j.jclinepi.2020.06.025 [DOI] [PubMed] [Google Scholar]
- 21.Knezevic NN, Candido KD, Vlaeyen JWS, Van Zundert J, Cohen SP. Low back pain. Lancet. 2021;398(10294):78-92. doi: 10.1016/S0140-6736(21)00733-9 [DOI] [PubMed] [Google Scholar]
- 22.Hayden JA, Ellis J, Ogilvie R, Malmivaara A, van Tulder MW. Exercise therapy for chronic low back pain. Cochrane Database Syst Rev. 2021;9(9):CD009790. doi: 10.1002/14651858.CD009790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rubinstein SM, de Zoete A, van Middelkoop M, Assendelft WJJ, de Boer MR, van Tulder MW. Benefits and harms of spinal manipulative therapy for the treatment of chronic low back pain: systematic review and meta-analysis of randomised controlled trials. BMJ. 2019;364:l689. doi: 10.1136/bmj.l689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdel Shaheed C, Maher CG, Williams KA, Day R, McLachlan AJ. Efficacy, tolerability, and dose-dependent effects of opioid analgesics for low back pain: a systematic review and meta-analysis. JAMA Intern Med. 2016;176(7):958-968. doi: 10.1001/jamainternmed.2016.1251 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol
eMethods. Content of the Interventions (TIDieR checklist)
eFigure 1. Timeline for intervention delivery
eReferences
Data sharing statement