Abstract
The Research Domain Criteria (RDoC) project’s success rests on the assumption that constructs and data can be integrated across units of analysis and developmental stages. We adopted a psychoneurometric approach to establish biobehavioral liability models of sensitivity to social threat, a key component of potential threat that is particularly salient to the development of adolescent affective psychopathology. Models were derived from measures across four units of analysis in a community sample (N=129) of 11-to-13-year-old girls oversampled for shy/fearful temperament. To test the ecological validity of derived factors, they were then related to real-world socio-affective processes in peer interactions over a 16-day ecological momentary assessment (EMA) protocol. Our results indicate that measures (i.e., amygdala reactivity to negative social feedback, eye-tracking bias toward social threat, parent- and adolescent-reports of social threat sensitivity) formed unit-specific factors, rather than one unified factor. These findings suggest that these factors were largely unrelated. Amygdala response to social punishment and attention bias toward threatening faces predicted real-world experiences with peers, suggesting that vigilance toward potentially threatening social information could be a mechanism through which vulnerable youth come to experience their peer interactions more negatively. We discuss measurement challenges confronting efforts to quantify developmentally sensitive RDoC constructs across units of analysis.
Keywords: RDoC, potential threat, fMRI, eye-tracking, ambulatory assessment, psychoneurometrics
General Scientific Summary
Sensitivity to social threats may be a key mechanism in adolescence that initiates and maintains life-long trajectories of affective psychopathology. We combined four different measures of social threat sensitivity (amygdala reactivity to negative social feedback, eye-tracking bias toward social threat, parent- and adolescent-reports of threat sensitivity). We show that vigilance toward threatening social information could be a mechanism through which vulnerable youth come to experience their peer interactions more negatively, and discuss measurement challenges confronting efforts to quantify developmentally sensitive constructs across units of analysis.
Complex social and neurobiological changes of adolescence contribute to heightened sensitivity to social threat, which confers risk for affective psychopathology during this developmental period (Kupferberg et al., 2016; Silk et al., 2012a). Sensitivity to social threat is particularly important to study in adolescent girls, who are more sensitive to social feedback (Hankin et al., 2007), and at higher risk for anxiety and depression relative to males (Merikangas et al., 2010). Although social threat can be studied across several different units of analysis (e.g., questionnaire, physiological, neuroimaging), researchers rarely integrate across units, which calls into question the coherence and validity of this construct.
Integrating findings across different units of analysis to potentially form a unified construct is a central goal of the National Institute of Mental Health’s Research Domain Criteria initiative (RDoC; Kozak & Cuthbert, 2016). Little research exists, however, on the utility of the RDoC framework for adolescent samples. Further, there is a need to consider essential developmental processes within the RDoC framework, to deepen our understanding of developmental mechanisms that may contribute to vulnerability for affective disorders (Franklin et al., 2015).
The present study addresses these limitations specifically in the RDoC construct of “potential threat” in the social environment, located at the intersection of the Negative Valence and Social Processes domains. We leverage Psychoneurometrics (Patrick et al., 2013) to develop biobehavioral factors of sensitivity to social threat that integrate multiple units of analysis, including developmentally informed and ecologically valid laboratory paradigms. To test the ecological validity of these factors, we used them to predict real-world socio-affective processes during daily peer interactions.
Dispositional Sensitivity to Social Threat
Adolescence is a developmental period characterized by considerable neurobehavioral and socio-affective development spurred by the onset of puberty as well as cognitive and social-contextual changes (Blakemore, 2008). It is associated with normative increases both in the salience of peer relationships and the frequency of negative peer events (Hankin et al., 2007), with particular increases in interpersonal stressors in adolescent girls (Rudolph & Asher, 2000). Coinciding with these socio-affective challenges is increased neural sensitivity to peer exclusion (Guyer et al., 2009). Because heightened sensitivity to social exclusion and rejection is to some degree developmentally normative yet does not affect all adolescents similarly, it is important to identify individual differences that moderate adolescents’ distress in response to social exclusion (Masten et al., 2009).
Although social threat sensitivity has been studied at different units of analysis (Silk et al., 2012b), developmental psychopathology research is often characterized by correlational relationships between disorder or trait-liability constructs and single neurobiological or psychophysiological outcomes (Patrick et al, 2013). Further, because socio-affective tasks used to elicit neural or physiological responses are administered in the lab, they have been criticized for low ecological validity (Rothbart & Bates, 2006). One way to address these limitations is to examine how measures of sensitivity to social threat across units of analysis relate to each other and to real-world socio-affective functioning. Thus, complementary research efforts that have conceptualized social threat sensitivity in neural, behavioral, parent- and self-report terms need to be integrated into one common metric.
One relevant line of research on social threat processing examines cognitive factors, such as attention bias toward threat, which is most often measured using the Dot Probe Task (DPT). Temperamentally fearful or shy children and adolescents at temperamental risk for anxiety (Pérez-Edgar et al., 2010) display an initial attention bias toward socially threatening stimuli. Adolescents with clinical levels of social anxiety disorder have demonstrated both initial hypervigilance and longer initial fixation duration on socially threatening stimuli compared to youth without social anxiety (Capriola-Hall et al., 2020). Such patterns of attention may contribute to the onset and maintenance of anxiety symptoms via overestimations of the likelihood and imminence of potential harm (Cisler & Koster, 2010). Research in adults has found positive associations between self-reported rejection sensitivity and attentional avoidance of threatening faces on the DPT (Berenson et al., 2009); to our knowledge, this has not been shown in adolescents.
Functional magnetic resonance imaging (fMRI) methods are also increasingly used to study sensitivity to social threat. However, traditional tasks used to study neural substrates of social processing, such as passive viewing of facial stimuli, provided little real-world relevance. Recent paradigms have improved ecological validity; for example, by simulating peer interactions on an online social platform (Guyer et al., 2008; Silk et al., 2012b). Given the high frequency of social media use, fMRI tasks with interactive platforms through which adolescents believe they are receiving positive or negative feedback from “real” peers provide ecologically valid approaches to studying neural sensitivity to social threat. Findings from these tasks may better approximate how adolescents are actually responding to social threats in the real world, which is supported by initial research linking heightened neural activity to social rejection and rejection sensitivity in adolescence (Burklund et al., 2007; Masten et al., 2009).
Neuroimaging data from virtual peer interaction tasks suggest that dispositional threat sensitivity may be mediated by hyperactivity in areas of an “affective salience network” (Masten et al., 2009; Silk et al., 2014), particularly the amygdala, in response to socially threatening stimuli (Monk et al. 2008; Pérez-Edgar et al., 2007). Cross-sectional studies suggest that increased sensitivity to anticipated peer evaluation rises in adolescence, and among girls in particular (Guyer et al., 2009; Rudolph & Flynn, 2014). Activity in the amygdala, and other regions associated with processing affective salience, to peer rejection increases with puberty and is elevated among youth with depression (Silk et al., 2014), and among youth reporting elevated levels of loneliness and rejection sensitivity (Burklund et al., 2007; Spithoven et al., 2017).
Further, ecological momentary assessment (EMA), which involves repeated sampling of emotions, thoughts, or behaviors in daily life, can be used to capture perceptions of social threat with high real-world relevance. EMA research in adolescent samples suggests that experiencing more social threat in daily life is associated with more negative mood states (Rusby et al., 2013). Only a few studies have linked EMA to neural measures of socioemotional processing in youth (Masten et al., 2012; Forbes et al., 2012; Silk et al., 2012; Price et al., 2016b; Oppenheimer et al., 2019). For example, work by Masten and colleagues (2012) suggests that more time spent with peers, measured using EMA, can potentially serve as a protective factor to blunt neural sensitivity to social threat over time. Additionally, Price et al. (2016b) linked brain activity, EMA, and attentional patterns in a sample of youth with anxiety disorders. The authors showed that greater vigilance towards threat on the DPT was associated with greater use of distraction and suppression techniques in response to negative events in daily life. Further, altered patterns of functional connectivity between the amygdala and prefrontal cortex mediated this link.
These findings suggest that social threat sensitivity may be assessed across different units of analysis as suggested by the RDoC framework, and that threat sensitivity is meaningfully related to real-world interpersonal experiences. Given the methodological challenges inherent in relating neurophysiological measures to psychological attributes, however, little research has directly examined how and whether these multiple methods could be integrated into a coherent construct of sensitivity to social threat, and how resultant factors are associated with with real-world social behaviors.
Multimodal Modelling of Sensitivity to Social Threats
Integrating units of analysis poses methodological challenges that require a suitable conceptual and analytic framework to address. Campbell and Fiske (1959) formalized the notion that valid constructs should converge across multiple methods of measurement while diverging from other constructs within a multitrait-multimethod matrix (Patrick et al. 2013). That is, measures include variance related to substantive constructs as well as their assessment modalities. A key implication is that measures often covary less when operationalized using different methods. Thus, sufficiently powered studies on links between neurophysiological and subjective report variables return modest effect sizes (Yancey et al., 2016).
Indeed, weak associations across measurement domains may account for the limited success of linking clinical symptomatology to biobehavioral constructs (Miller & Rockstroh, 2013). This is the fundamental challenge facing RDoC, which seeks to generate nomological networks for constructs that span units of assessment. Using the multitrait-multimethod approach to understand the elements of the RDoC matrix as a network of observations, constructs, and interpretative rules may prove fruitful in addressing these issues (Kozak & Miller, 1982).
The construct-network, or psychoneurometric, approach proposed by Patrick et al. (2013) is one way to address this challenge. It is grounded in the classic psychological assessment theory and methodology discussed above, which attributes systematic variability in scores to distinct influences operating within and across particular measurement modalities (Cronbach & Meehl, 1955; Patrick et al., 2013). In essence, psychoneurometrics use classic measurement development techniques to establish multi-modal measures of constructs that span units of analysis. The focus is on biobehavioral traits that are hypothesized to be associated with distinct neurobehavioral systems, representing a good match to the logic and goals of the RDoC framework.
Notably, some quantitative measurement models integrating measures of threat sensitivity across different units of analysis already exist in adult populations. Threat sensitivity has, for instance, been conceptualized as proneness to react to acute aversive stimuli (Yancey et al., 2016), predicting both clinical and neurophysiological criterion measures such as social phobia, general corrugator muscle tension, or specific event-related potentials. Similarly, the predictive validity of composites of questionnaires and neurophysiological indicators of threat sensitivity has been extended to real-life outcomes, such as suicidality (Venables et al., 2018). Kramer and colleagues (2012) modeled the structure of various existing questionnaire measures of situational fear- and fearless-dominant tendencies in a large adult twin sample and identified a factor that accounted for substantial variance in all scales and was associated with variations in aversive startle potentiation (Lang et al., 2000). Of these related previous studies, however, none have focused on social aspects of threat, which are most relevant to adolescent development.
Some complements to these dispositional constructs in adults exist in the developmental literature. Moser et al. (2015), for example, discussed preliminary psychoneurometric findings based on a sample of preschoolers, emphasizing the usefulness of a transdiagnostic approach to incorporating physiological measures in developmental assessments, the authors also emphasize that this represents an underused approach in need of further testing and specification.
Psychoneurometrics epitomize a promising methodological approach to understanding the elements in the RDoC matrix as a set of indicators, constructs, and interpretative rules that set them in relation to one another. Considered in this way, delineating biobehavioral constructs based on RDoC traits and exploring how they interface with developmentally salient real-life experiences is essential to an understanding of the role that psychological processes and underlying neurobiological systems play in the development of youth psychopathology.
The Current Study
Social threat sensitivity represents a developmentally sensitive biobehavioral attribute, expected to confer liability for interpersonal problems with peers in real life. To test this hypothesis, we use a neurodevelopmentally-informed, multimodal-assessment perspective for the operationalization of individual differences in sensitivity to social threat (i.e., psychoneurometrics; Patrick et al., 2013). This approach is akin to the construction of psychometric scales, where items are selected in iterative data collection and analysis, gradual modification of scales, and constituent items, through analysis of their internal associations and relations with conceptually relevant criterion measures (Miller & Rockstroh, 2013).
We focus our efforts on early adolescent girls high in shy/fearful temperament, given the high risk of future affective psychopathology in this population. Consistent with the RDoC strategy, we explore whether sensitivity to social threat measured across multiple units represents a coherent biobehavioral disposition, or is better captured by independent unit-specific factors.
We then test whether this model is significantly linked to real-world socio-affective functioning measured using EMA. The work reported here is the first to model relations of multiple indicators from both neurobiological and eye-tracking indices with trait-scale measures of developmentally-sensitive constructs and set them in relation to real-world momentary socio-affective processes, such as feelings of social connectedness and negative affect (NA) during peer interaction.
Method
All study procedures were approved by the University of Pittsburgh Institutional Review Board (STUDY19070027). Participants were recruited for a longitudinal study of risk for anxiety and depression in adolescent girls via community advertisements. Informed consent and youth assent were obtained after a detailed study explanation. Measures used here were obtained during three separate visits to the lab: The first visit comprised completing clinical interviews and self- and parent-reports, the second involved completing the DPT and the third involved completing fMRI tasks. EMA was collected at home between the second and third visits.
Participants
Participants were 129 girls (Mage=12.28, SDage=.80; detailed overview in supplement), of which 65% were white, 20% black/African American, 2% Asian, 9% biracial, 1% Native American, and 1% other.
Measures
Adolescent-Report
The Pubertal Development Scale (PDS; Petersen et al., 1988) measures gonadal (e.g., breast development, menarche) and adrenal (e.g., skin changes) indicators of physical development. Response options range from 1 (no) to 4 (development seems complete), and in accordance with Shirtcliff et al. (2009) separate adrenarche and gonadarche scores were computed based on specific items of the PDS.
The Loneliness and Social Dissatisfaction Questionnaire (LSDQ; Asher & Wheeler, 1985) assesses feelings of loneliness, appraisals of current peer relationships, perceptions of the degree to which important relationship provisions are being met, and perceptions of social competence. The 16 primary items are summarized to represent a sum score and are rated on a scale from 1 (no), 2 (sometimes) to 3 (yes).
The Fear of Negative Evaluation Scale (FNE; Collins et. al, 2005) is a measure of rejection sensitivity and attitudes toward social evaluation, consisting of 12 items. Response options range from 1 (not at all) to 5 (extremely).
The five-item victimization subscale of the Peer Relations Questionnaire (PRQ; Rigby & Slee, 1993) was used as a measure of perceived social, physical, and verbal peer victimization, with scores ranging from 5 (low peer victimization) to 20 (high peer victimization).
Parent-Report
We used the fearfulness and shyness subscales of the revised parent version of the Early Adolescent Temperament Questionnaire (EATQ-R; Ellis & Rothbart, 2001). Fearfulness refers to the tendency to experience unpleasant affect related to anticipation of distress, and shyness refers to behavioral inhibition to (social) novelty and challenge. Each item is answered on a five-point Likert scale ranging from 1 (almost never true) to 5 (almost always true).
Parents further reported on the 15-item sensitivity to punishment subscale of the Sensitivity to Punishment/Sensitivity to Reward Questionnaire (SPSR; Colder et al., 2011), pertaining to fear/shyness and anxiety. Items were rated on a five-point Likert scale, ranging from 1 (strongly disagree) to 5 (strongly agree).
Attention Bias
Participants completed a modified version of the computerized Dot Probe Task (DPT, MacLeod et al., 1986). Stimuli for this task were photographs of pairs of male and female faces of varied races from a standardized set (Tottenham et al., 2009). Each trial (128) began with the presentation of a central fixation cross, followed by paired facial stimuli presented for 1000 ms. After the faces disappeared, a probe appeared (one or two asterisks) in the location of either the emotional or the neutral face.
The derived set of indicators represents standard gaze pattern measures of key attentional components (Cisler & Koster, 2010) relevant to anxiety and depression, both of which are hypothesized to be closely related to social threat sensitivity. We primarily relied on eye-tracking indices of gaze position to assess attention bias, which provide a more reliable indicator than traditionally used reaction times (Price et al., 2013). Gaze data were measured using the Tobii T60XL eye-tracking monitor (sampling rate of 60 Hz; Tobii Technology, Inc., Falls Church, VA). Accuracy of calibration was determined by a research assistant. Tobii’s standard fixation filter (I-VT) was applied to classify fixations, and areas of interest (AOI) around the facial stimuli were created in order to determine the location of each fixation.
Consistent with prior research (Capriola-Hall et al., 2020; Rosen et al., 2019), initial vigilance to threat was defined as the time taken to look at neutral face relative to the time taken to look at an angry face (i.e., latency bias score). To measure sustained attentional capture, we calculated a fixation bias score (i.e., fixation time on threatening face – fixation time on neutral face; Cisler & Koster, 2010).
Previous work has also used similar indices as the current study to assess attention at later stages, comparing the fixation duration on threatening faces relative to neutral faces (Price et al., 2016a) and examined difficulty disengaging attention by comparing time to look away from emotional faces compared to time to look away from neutral faces (Chen et al., 2012). Therefore, as a measure of difficulty disengaging, we assessed time taken to look away from the angry face and fixate on the probe that appears in the opposite location of where the negative face was presented (time to disengage), compared to time taken to look away from the neutral face and fixate on the probe that appears in the opposite location of where the neutral face was presented (i.e., time to disengage from threatening face – time to disengage from neutral face; Cisler & Koster, 2010).
Peer Social Incentive Delay (P-SID) Task.
The P-SID task, which is a social adaptation of the original Monetary Incentive Delay task (MID; Knutson et al., 2000), was designed to measure brain activity related to social rewards and punishments. We created a new “peer observation” version of the task to examine neural activation to anticipation and receipt of social feedback from a virtual peer based on the participants’ task performance. To enhance the ecological validity of the task, participants were told that other girls participating in the study at other sites would be watching them complete the P-SID task and providing feedback after each trial by pressing a button to send a smiling or frowning picture of themselves. In addition, prior to the scan, participants were asked to evaluate the performance of one of these virtual peers by sending them a smiling or frowning picture of themselves to signal positive and negative feedback of the virtual peer’s performance. While they served as evaluator, participants were told that the video feed was one of the girls who was performing the P-SID task at another site and for whom the participant was serving as evaluator. Figure 1 depicts the trial structure of the P-SID along with an overview of the three task conditions.
Figure 1.
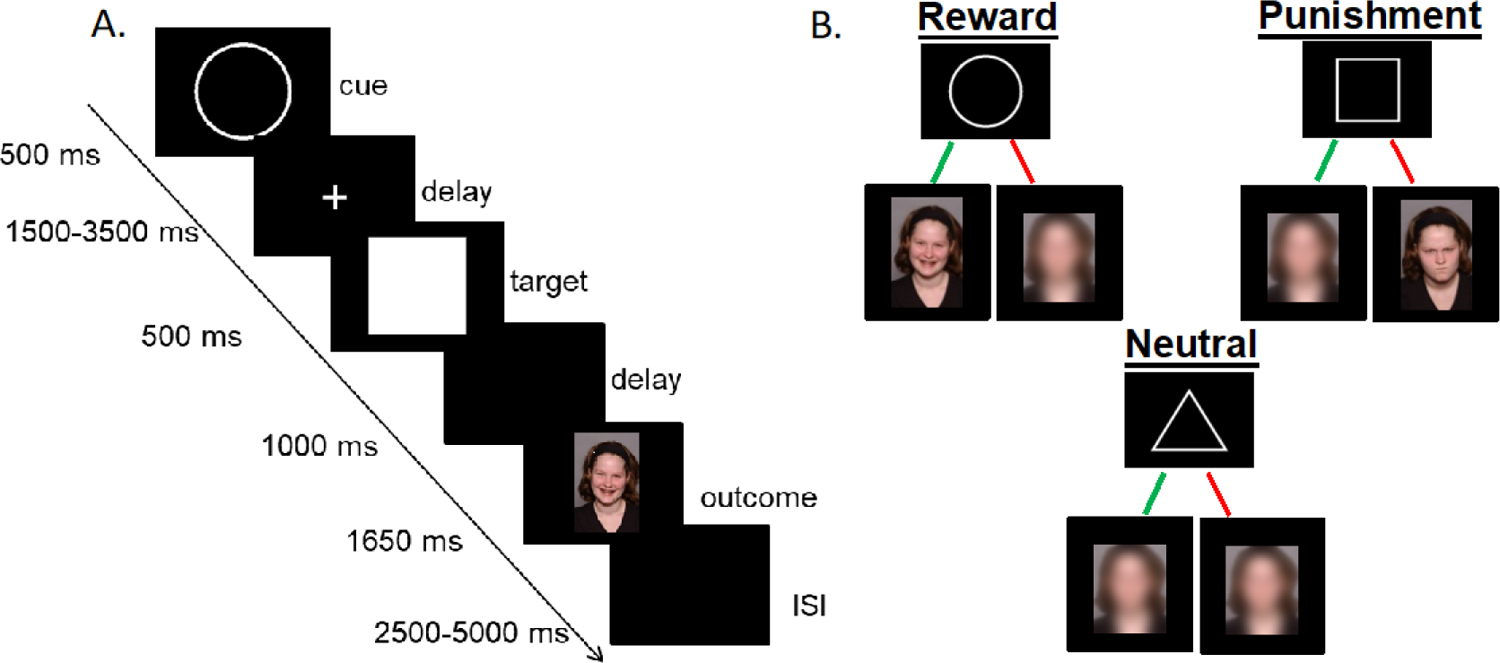
P-SID task and feedback conditions. A: Trial Structure. An initial cue (500 ms) and fixation (1500–3500 ms) cross were first presented for each trial. Participants were instructed to press a button as fast as possible when the target displayed on the screen. Following the target, a black screen was displayed (1000 ms) and the feedback was presented (1650 ms). A second black screen followed the feedback prior to the next trial (2500–5000 ms); B: The task consisted of 72 trials (27 social reward, 25 social punishment, 18 control).
For the first-level analyses, individual effects were estimated using the general linear model approach implemented in SPM12. Group analyses focused on a mask that was constructed by combining meta-analytic maps for the terms social (z>3.30, FDR p<.05) and punishment (z>4.45, FDR p<.05) from Neurosynth.org. Analyses were conducted using AFNI’s 3dttest with the -Clustsim option, which uses a nonparametric approach to cluster-size thresholding with a cluster forming threshold of p<.001, extent threshold ~23 voxels. For parsimony, we extracted parameter estimates from the punishment receipt>neutral contrast in three sub-regions of the amygdala (laterobasal nuclei (LB), the superficial nuclei (SF), and the centromedial nuclei (CM)), which were anatomically defined using the SPM Anatomy Toolbox, v.13. (Table S1). Task-related activation is reported elsewhere (Ladouceur et al., in prep.) but we include a description of the task-related activation for the punishment receipt>neutral in the supplementary materials (see Table S2).
These nuclei broadly resemble the microanatomy and connectivity of amygdala nuclei across mammalian species (McDonald, 1998). The LB nuclei include the lateral, basolateral, basomedial, and basoventral nuclei, which facilitate fear learning processes through connections with cortical (e.g., prefrontal cortex) and subcortical (e.g., hippocampus) regions (Klavir et al., 2017). The SF nuclei includes cortical nuclei and may be involved in affective (Roy et al., 2009) and social processes (Goossens et al., 2009). The CM nuclei play an important role in generating the behavioral, autonomic and motor responses to salient information. Animal studies have shown dense connections between the CM nuclei and brainstem, hypothalamic and basal forebrain regions (Fudge and Haber, 2000; Ghashghaei et al., 2007). While other neural regions are implicated in processing social threat, we focus on subregions of the amygdala as a preliminary step that will inform future work in this area. FMRI stimuli, Data Acquisition and Preprocessing are detailed in the supplementary materials.
Momentary Negative Affect and Connectedness with Peers
Ecological Momentary Assessment.
Participants answered questions on a smartphone for 16 consecutive days (2 school weeks, 3 weekends) prior to the fMRI visit. They were randomly sampled within 3 blocks of time on weekdays (morning, after school and evening) and 4 blocks of time on weekends (morning, early/late afternoon, evening), for a total of 54 samples. They responded to a 3–5-minute series of prompts about mood and social context (Silk et al., 2011) using a study-provided smartphone that includes WebDataExpress. Participants indicated current social companions at the moment of the call, and were asked how close/connected they felt with those people using a “0” to “100” sliding scale. Participants were additionally asked to report on their most recent negative interaction with a peer since the last “beep” and to type out details about this interaction in a free response box, which allowed for quality checking. Participants were also asked to report on how “worried,” “stressed”, “mad”, and “sad” they felt following each interaction, again reported using a 0–100 slider. After-school assessment allowed youth to report on interactions that occurred during the school day.
All four momentary measures of NA were strongly intercorrelated (range of r =.51-.86), and were aggregated to obtain a global index of NA related to peer social interaction.
Data Analysis
In line with previous work on RDoC-based psychoneurometric models (Patrick et al., 2013) the following steps were followed: We first calculated zero-order correlations for hypothesized indicators of social threat sensitivity within measurement domain to map out empirical relationships among a priori selected indicators.
We then used confirmatory factor analysis to evaluate the fit of two alternative cross-domain models. A single cross-domain factor model, in which indicators from the four units of analysis were specified as loading directly on one factor was estimated first. We then tested a second model, in which indicators from the different units of analysis were specified as loading on four distinct unit-specific correlated factors.
Next, we introduced our EMA variables as outcomes to each model. The cross-domain factor model tested whether the shared variance among indicators from differing measurement domains was linked to momentary negative affect or interpersonal connectedness in daily peer-interactions, but not whether any of the four lower-order factors had unique effects. Therefore, by establishing four correlated factors in our second model, each defined by different sets of indicators from varying measurement domains, we tested whether each unit had unique predictive power of the EMA variables.
Variance in age, in contrast to pubertal status, was relatively low in our sample because girls were recruited to fall in approximately the same age range. Due to the restricted age range, we only include pubertal status as a covariate to control for developmental differences in our analyses.
We used full information maximum likelihood estimation to accommodate missing data for individual indicators. For each model, absolute fit was assessed using the χ2-test, which yields lower values for better fitting models, as well as root mean square error of approximation (RMSEA), standardized root mean square residual (SRMR), and comparative fit index (CFI). For RMSEA values ≤.05, for SRMR <.08, and CFI and TLI ≥ .95 indicate good fit (Hu & Bentler, 1999).
Results
Table 1 presents descriptive statistics and within-domain correlations of social threat sensitivity with psychometric scale, eye-tracking, and amygdala reactivity. Internal consistency reliabilities (α) for psychometric scale indicators fell in the range of .67 to .87, indicating good reliability of our subjectively reported trait composites. Spearman-Brown coefficients for the DPT scores ranged from .30 for the latency bias to .65 and .68 for the disengagement and fixation biases, indicating poor to moderate reliability.
Table 1.
Means, Standard Deviations, and Correlations Among Indicators of Social Threat Sensitivity Within Each Unit of Analysis.
| Indicator | M | SD | 1 | 2 | 3 |
|---|---|---|---|---|---|
| Parent-report (N=118–127) | |||||
| 1. EATQ (fear) | 2.33 | 0.73 | 1 | ||
| 2. EATQ (shyness) | 2.74 | 0.98 | .19 [.02,.36] | 1 | |
| 3. SPSR (threat sensitivity) | 2.65 | 0.52 | .46 [.30,.59] | .48 [.33, 61] | 1 |
|
| |||||
| Adolescent-report (N=126–127) | |||||
| 1. FNE | 32.1 | 5.86 | 1 | ||
| 2. LSDQ | 22.83 | 3.64 | .22 [.05,.38] | 1 | |
| 3. PRQ (victimization) | 6.86 | 2.09 | .25 [.08,.41] | .47 [.32,.60] | 1 |
|
| |||||
| Dot Probe Task (N=118–129) | |||||
| 1. fixation | 64.56 | 67.91 | 1 | ||
| 2. latency | −136 | 306.67 | −.32 [−.48,−.15] | 1 | |
| 3. disengagement | −114.91 | 341.22 | −.25 [−.41,−.07] | .73 [.64,.80] | 1 |
|
| |||||
| Peer Social Incentive Delay Task (N=87) | |||||
| 1. right LB amygdala | −0.08 | 1.26 | 1 | ||
| 2. left LB amygdala | −0.02 | 1.4 | .62 [.46,.73] | 1 | |
| 3. left SF amygdala | 0.21 | 3.28 | .48 [.30,.63] | .78 [.68,.85] | 1 |
| 4. right SF amygdala | 0.17 | 2.87 | .56 [.40,.69] | .66 [.51,.77] | .83 [.75,.89] |
Note. Values in square brackets indicate the 95% confidence interval for each correlation. FNE=Fear of Negative Evaluation Scale; LSDQ=Loneliness and Social Dissatisfaction Questionnaire; PRQ=Peer Relationships Questionnaire (victimization subscale); EATQ=Emotions and Temperament Questionnaire (fear/shyness subscales); SPSR=Sensitivity to Punishment/Sensitivity to Reward Questionnaire; DPT=Dot Probe Task; LAT, FIX, and DIS denote different bias scores derived from the DPT; Social Incentive Delay Task: amygdala activation in the left vs. right hemisphere in response to negative social feedback; LB / SF=laterobasal and superficial subdivisions of the amygdala.
Correlations among eye-tracking bias scores were modest to high (range of r = |.25|-|.73|), and loaded onto a threat bias factor, such that higher threat bias was associated with a shorter latency to attend to threat, faster disengagement, and greater fixation time on threat faces, which suggests re-orienting towards threatening faces after initial disengagement. Parent- and adolescent-reports were moderately intercorrelated (range of r = .19-.48), whereas correlations among amygdala subdivisions were high in magnitude (range of r = .48-.83). Cross-domain associations were weak, with a mean correlation of r = .09.
Cross-Domain Models of Social Threat Sensitivity
The one-factor model indicated a poor fit to the data: χ2(64)=292.01, p<.001, RMSEA=.166, CFI=.48, TLI=.36; SRMR=.177. Of the thirteen indicators, only the amygdala ROIs (left and right LB and SF) and the victimization subscale of the PRQ showed significant factor loadings onto a single common factor. In contrast, the alternative four unit-specific factor model fit the data well: χ2(58)=71.288, p =.112, RMSEA=.042; CFI=.97; TLI=.96; SRMR=.054. The sensitivity to punishment subscale (SPSR) showed the strongest loadings on the parent-report factor, while self-reported rejection sensitivity demarcated the strongest loadings on the adolescent-report factor. Among the amygdala ROIs, the highest factor loading was observed for the right LB. Loadings of the three eye-tracking bias scores on the attention bias factor were in the same range. Of the four latent factors, only the ones comprising adolescent-reports and eye-tracking indices of attention bias to threat were significantly linked to each other.
Cross-Domain Models Predicting Momentary Socio-Affective Peer-Processes
Because our one-factor model was characterized by a poor model fit, the EMA criterion measures were only introduced into the four-factor model. Higher scores on latent factors reflect higher levels of social threat sensitivity (Figure 2). Our momentary external validation criteria were not significantly associated with each other (r=0.13, p=0.372). Scores on the adolescent-report factor showed positive associations with momentary peer-related NA and strong negative associations with feelings of social connectedness during peer-interactions. In addition, the factor reflecting interrelated portions of variance among amygdala ROIs had an incremental predictive value for momentary connectedness, yet not for peer-related NA. Scores based on the DPT factor, in contrast, uniquely contributed to the prediction of momentary peer-related NA. For the parent-report factor, no significant associations with either of the outcomes emerged. Overall, this model showed a good fit: χ2(94)=107.11, p=.167, RMSEA=.033, SRMR =.057, CFI=.97, TLI=.95. None of the four latent factors were significantly associated with pubertal status subscales.
Figure 2.
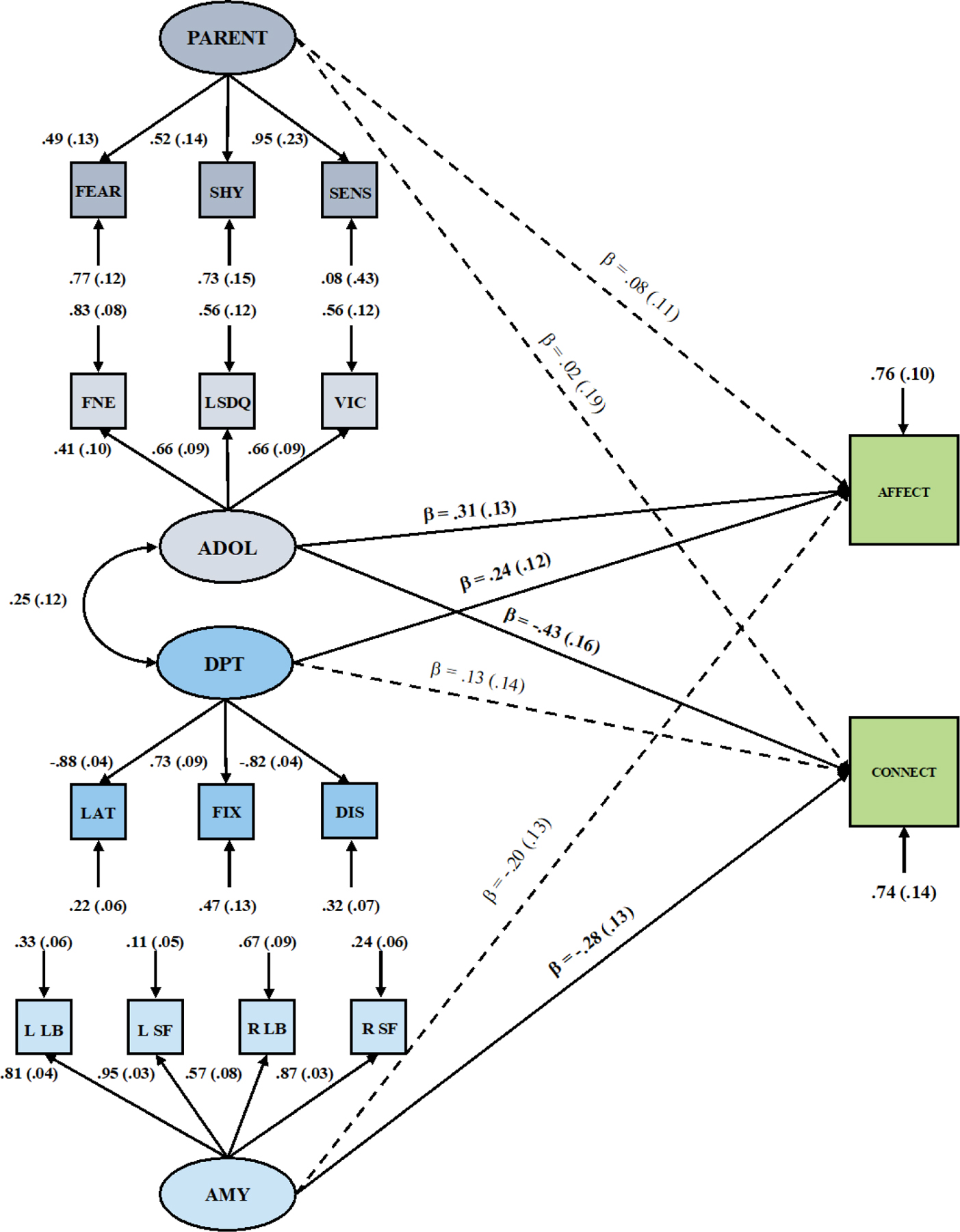
Four-factor model of social threat sensitivity predicting momentary negative affect feelings of connectedness after social interactions with peers. Standardized path estimates depicted. Solid lines denote significant, dashed lines non-significant regression paths at 95% CI; Single headed arrows indicate regression paths; double headed arrows indicate correlations, only significant covariance arrows are presented. ADOL=adolescent; FNE=Fear of Negative Evaluation Scale; LSDQ=Loneliness and Social Dissatisfaction Questionnaire; VIC=victimization subscale of the PRQ; Fear/Shy=fear and shyness subscales of the EATQ; SENS=threat sensitivity subscale of the SPSR; DPT=Dot Probe Task; LAT, FIX, and DIS denote different bias scores derived from the DPT; AMY=amygdala; L=left hemisphere; R=right hemisphere; LB/SF=laterobasal and superficial subdivisions of the amygdala; Coefficients for covariates are not presented for parsimony.
Discussion
We leveraged the psychoneurometric approach to evaluate and integrate developmentally sensitive RDoC constructs measured at different units of analysis. We first developed models of sensitivity to social threat that cut across RDoC units of analysis, and found that method-specific variance overshadowed construct specific variance, resulting in a four-factor structure of largely independent, unit-specific factors with adolescent-report and attention bias being the only factors that were weakly associated. Both, the attention bias and amygdala factors accounted for separate portions of variance in our EMA outcomes beyond adolescent-reports, as evidenced by significant beta coefficients for both when entered as predictors in structural equation models together with adolescent-reports.
Although RDoC aspires for an integrated heterophenomenological approach (MacNamara, & Phan, 2016), whether this is feasible or not for any given construct/domain remains an open question. When a single-factor model fails to emerge, estimating a full model with all available unit-specific factors (here four correlated factors) offers several benefits, including concurrently examining relationships among units, as well as emphasizing the unique associations between specific units in the prediction of a criterion variable. Using SEM to simultaneously estimate the effects of multiple units as latent constructs provides more reliable estimates of each unit, and is distinct from traditionally employed bivariate-mapping approaches, which often use one measure of a hypothesized construct as outcome and treat it as what Patrick et al. (2019, p. 1514) call an “indisputable, unmodifiable criterion” to which indicators from other units of analysis are scaled. Consequently, measures across modalities are not sufficiently integrated within the hypothesized biobehavioral trait framework. Thus, even in the absence of a hypothesized unified factor, with four correlated second-order factors, we arrive at construct approximations of social threat sensitivity that reflect systematic variance in multiple domains when looking across the factors, instead of isolated unit-specific indicators in separate models.
We demonstrate a direct link between adolescent-reported social threat and eye-tracking indices of attention bias. Beyond the validation of this laboratory task with real-world outcomes, this finding suggests that biased attention towards threatening stimuli may play a role in how shy/fearful adolescent girls process their interactions with peers in daily life. Girls with biased attention towards threatening stimuli may be more likely to attend to and recall negative interactions with peers and/or interpret peer interactions more negatively.
The relationship of amygdala reactivity with peer connectedness replicates previous findings in adults, showing that those with greater activation in regions associated with processing threat (i.e., ACC, amygdala, periaqueductal grey) during a social rejection task reported feeling greater momentary social distress in daily life (Eisenberger et al., 2007). In this study we included three bilateral subregions of the amygdala, which broadly resemble the microanatomy and connectivity of amygdala nuclei across mammalian species (McDonald, 1998). Of these subregions, left and right LB and SF left and right loaded onto a factor with right LB having the strongest loading. The LB nuclei include mostly glutamatergic projection neurons that receive sensory inputs from the thalamus, insula, and sensory association cortices as well as from the medial prefrontal cortex (Felix-Ortiz et al., 2013; Sah et al., 2003). LB nuclei play an important role in anxiety (Etkin et al., 2009) and based on a study in primates, they are involved in suppression of social behavior (Wellman et al., 2016).
Overall, these findings suggest that the neurobiology supporting heightened sensitivity to social-evaluative threat in adolescent females is associated with real-world socio-affective processing. Further, aligning with previous theoretical work (Silk et al., 2012a), sensitivity to social threat may be amplified in shy/fearful adolescents; to validate the clinical utility of our model, it remains to be tested whether this profile could confer risk for future anxiety or depression.
The strongest predictor of momentary peer processes was adolescent-report, highlighting that, although neurobiological measures emphasized in the RDoC initiative add some incremental value, subjective experience remains a critical factor in the assessment of developmentally sensitive interpersonal processes. However, the criteria used to validate our models were based on repeated self-report measures of adolescents’ affect and experiences of connectedness in daily life. Therefore, although EMA data offers increased insight into naturalistic conditions of peer relationships and reduces retrospective bias, we acknowledge that it also shares substantial method variance with adolescent-reports on dispositional attributes, likely inflating the observed relationship.
These results are in line with a recently published meta-analysis (Clarkson et al., 2020), suggesting that NIMH-funded studies have yet to provide strong support the RDoC framework in youth. Importantly, the psychoneurometric framework and research on the RDoC constructs and subconstructs was derived from studying adult populations, and that adults are considered the model system. Therefore, little is known about whether the correspondence between units of analysis observed in adults extend to youth. Generalizing from past studies in adults to youth samples invokes some uncertainty, because reliability – and thereby the portion of variance attributable to the construct of interest – is a function of task characteristics, administration, and the respondents assessed (Dang et al., 2020). Consequently, the lack of factorial uniformity among methods raises both conceptual and methodological questions about the feasibility of data integration of the RDoC’s units of analysis in youth samples.
Conceptually, the fact that the four-, instead of a unified-factor model showed a better fit to our data, casts doubt on whether average action tendencies as measured by questionnaires and behavioral task performance are shared functional components in adolescent girls. That unifactorial models have been estimated in adult samples suggests the possibility of individual indicators becoming integrated over time, a process known as dedifferentiation, discussed in research on specific cognitive abilities that become increasingly correlated throughout development (de Frias et al., 2007). However, support for this proposition has been mixed, and although any deviation from adult data may likely be interpreted in terms of immaturity, it is important to recognize that differences in measurement properties across age groups may yield a pattern of associations that could be falsely attributed to dedifferentiation.
Thus, weak correlations across units of analysis may also result from poor reliability of behavioral measures and distinct response processes involved in the two measurement types (Dang et al., 2020). For instance, support for dedifferentiation would also emerge if there were particular kinds of measurement errors that systematically co-occur with specific age groups. It is well-known from behavioral or fMRI tasks that age is significantly correlated with performance (see Luna et al. (2010) for an overview). From that perspective, it is reasonable to assume that individual tests could become more closely related to each other with age, because each test would show progressively less test-specific or error variance, and increased portions of construct specific variance (e.g.,Karr et al., 2018). This has immense implications for the development of multimodal study protocols, because it suggests that only limited amounts of construct-specific variance can be captured by a latent factor due to participant age and task reliabilities.
Relatedly, fMRI scans are highly sensitive to movement artifacts, and >20% of our sample had to be excluded from fMRI analyses due to head motion. This implies that tasks purporting to measure hypothesized RDoC constructs will require recruiting more youth participants in order to obtain sufficient numbers of subjects in young age groups. It also suggests that establishing standard, age-appropriate batteries of RDoC units of analysis is key to effectively reduce false-positive findings regarding correlations between units of analysis (Clarkson et al., 2020), while maximizing the capacity of the tasks to detect construct-relevant variance.
Another important notion from our data with particular relevance for research settings with younger samples that are more likely to include informant reports, is that adolescent- and parent-report were not significantly related, and parent-report did not contribute to the prediction of our momentary outcomes. This finding should be interpreted in the context of previous work indicating particularly high levels of informant discrepancies between parent- and adolescent-reports on questions about peer relationships (Kraemer et al. 2003). However, weak associations among parent- and adolescent-reports represent a robust observation in youth mental health research (Kraemer et al. 2003), with correspondence levels among informants being so low that no one informant’s subjective report is substitutable with another’s.
Although we believe that this study constitutes a meaningful step towards the integration of development into the RDoC framework and an important extension of previous work, our results point to crucial challenges accompanying the use of psychoneurometrics, such as low reliability and fractional trait-related variance of neurobehavioral indicators, along with the question of how selectively neurophysiological indicators index a specific attribute of interest as opposed to others (Patrick et al., 2013). The psychoneurometric framework was derived from unique model specifications, which runs the risk of basing inferences on sample characteristics rather than providing generalizable patterns. We therefore emphasize that the model we report reflects a highly specific conceptualization of sensitivity to social threat, based on a sample of early adolescent girls oversampled for fearful temperament. This sample was chosen to represent a developmentally sensitive period of hypervigilance to peer rejection, with good variability in threat responding. However, our model may not generalize to other populations of youths. Moreover, given the inductive and iterative nature of the psychoneurometric research strategy, mirroring the strategy of good measurement development, it is important to note that none of the reported correlations between subjectively reported attributes and biological measures can be viewed as definitive or comprehensive. Instead, this study offers an initial opportunity to test relevant measures and review the interrelations across units of analysis.
For these reasons, the results reported in this paper have to be viewed as the first step in a long-term strategy, explicitly requiring follow-up studies that replicate, extend, or revise our model, preferably with sophisticated longitudinal designs with multiple assessments of both biobehavioral and momentary outcomes to corroborate it. Models employing structural equivalence of derived factors across age or pubertal status are indispensable to examine the stability of these factors, and whether changes in these biobehavioral factors relate to changes in socioemotional processing.
We conclude that the use of biobehavioral measures capturing variation in prioritized detection and quick response to social threats, and rejection in particular, may provide a better understanding of the role that psychological processes and underlying neurobiological systems play in the development of youth psychopathology. Data derived from EMA protocols is a valuable source for the characterization of the functional significance of derived measures in daily life.
Supplementary Material
Acknowledgments
This study was supported by National Institutes of Health grants (R01 MH103241; UL1 TR001857).
References
- Asher SR, & Wheeler VA (1985). Children’s loneliness: A comparison of rejected and neglected peer status. Journal of Consulting and Clinical Psychology, 53(4), 500–505. [DOI] [PubMed] [Google Scholar]
- Berenson KR, Gyurak A, Ayduk Ö, Downey G, Garner MJ, Mogg K, Bradley BP, & Pine DS (2009). Rejection sensitivity and disruption of attention by social threat cues. Journal of Research in Personality, 43(6), 1064–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore SJ (2008). The social brain in adolescence. Nature Reviews Neuroscience, 9(4), 267–277. 10.1038/nrn2353 [DOI] [PubMed] [Google Scholar]
- Burklund LJ, Eisenberger NI, & Lieberman MD (2007). The face of rejection: Rejection sensitivity moderates dorsal anterior cingulate activity to disapproving facial expressions. Social Neuroscience, 2(3–4), 238–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell DT, & Fiske DW (1959). Convergent and discriminant validation by the multitrait-multimethod matrix. Psychological Bulletin, 56(2), 81–105. [PubMed] [Google Scholar]
- Capriola-Hall NN, Ollendick TH, & White SW (2020). Gaze as an Indicator of Selective Attention in Adolescents with Social Anxiety Disorder. Cognitive Therapy and Research, 44(1), 145–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen NT, Clarke PJ, MacLeod C, & Guastella AJ (2012). Biased attentional processing of positive stimuli in social anxiety disorder: An eye movement study. Cognitive Behaviour Therapy, 41(2), 96–107. [DOI] [PubMed] [Google Scholar]
- Cisler JM, & Koster EH (2010). Mechanisms of attentional biases towards threat in anxiety disorders: An integrative review. Clinical Psychology Review, 30(2), 203–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarkson T, Kang E, Capriola-Hall N, Lerner MD, Jarcho J, & Prinstein MJ (2020). Meta-analysis of the RDoC social processing domain across units of analysis in children and adolescents. Journal of Clinical Child & Adolescent Psychology, 49(3), 297–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colder CR, Trucco EM, Lopez HI, Hawk LW Jr, Read JP, Lengua LJ, Weiczorek, & Eiden RD (2011). Revised reinforcement sensitivity theory and laboratory assessment of BIS and BAS in children. Journal of Research in Personality, 45(2), 198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins KA, Westra HA, Dozois DJA, & Stewart SH (2005). The validity of the brief version of the Fear of Negative Evaluation Scale. Journal of Anxiety Disorders, 19(3), 345–359. [DOI] [PubMed] [Google Scholar]
- Cronbach LJ, & Meehl PE (1955). Construct validity in psychological tests. Psychological Bulletin, 52(4), 281–302. [DOI] [PubMed] [Google Scholar]
- Dang J, King KM, & Inzlicht M (2020). Why Are Self-Report and Behavioral Measures Weakly Correlated?. Trends in Cognitive Sciences, 24(4), 267–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Frias CM, Lövdén M, Lindenberger U, & Nilsson LG (2007). Revisiting the dedifferentiation hypothesis with longitudinal multi-cohort data. Intelligence, 35(4), 381–392. [Google Scholar]
- Egger HL, Pine DS, Nelson E, Leibenluft E, Ernst M, Towbin KE, & Angold A (2011). The NIMH Child Emotional Faces Picture Set (NIMH-ChEFS): A new set of children’s facial emotion stimuli. International Journal of Methods in Psychiatric Research, 20(3), 145–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis LK, & Rothbart MK (2001, April). Revision of the early adolescent temperament questionnaire. In Poster presented at the 2001 biennial meeting of the society for research in child development, Minneapolis, Minnesota. [Google Scholar]
- Etkin A, Prater KE, Schatzberg AF, Menon V, & Greicius MD (2009). Disrupted amygdalar subregion functional connectivity and evidence of a compensatory network in generalized anxiety disorder. Archives of General Psychiatry, 66(12), 1361–1372. [DOI] [PubMed] [Google Scholar]
- Felix-Ortiz AC, Beyeler A, Seo C, Leppla CA, Wildes CP, & Tye KM (2013). BLA to vHPC inputs modulate anxiety-related behaviors. Neuron, 79(4), 658–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forbes EE, Stepp SD, Dahl RE, Ryan ND, Whalen D, Axelson DA, Birmaher B, & Silk JS (2012). Real-World Affect and Social Context as Predictors of Treatment Response in Child and Adolescent Depression and Anxiety: An Ecological Momentary Assessment Study. Journal of Child and Adolescent Psychopharmacology, 22(1), 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin JC, Jamieson JP, Glenn CR, & Nock MK (2015). How Developmental Psychopathology Theory and Research Can Inform the Research Domain Criteria (RDoC) Project. Journal of Clinical Child & Adolescent Psychology, 44(2), 280–290. [DOI] [PubMed] [Google Scholar]
- Goossens L, Kukolja J, Onur OA, Fink GR, Maier W, Griez E, Schruers K, & Hurlemann R (2009). Selective processing of social stimuli in the superficial amygdala. Human Brain Mapping, 30(10), 3332–3338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, Lau JYF, McClure-Tone EB, Parrish J, Shiffrin ND, Reynolds RC, Chen G, Blair RJR, Leibenluft E, Fox NA, Ernst M, Pine DS, & Nelson EE (2008). Amygdala and Ventrolateral Prefrontal Cortex Function During Anticipated Peer Evaluation in Pediatric Social Anxiety. Archives of General Psychiatry, 65(11), 1303–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer AE, McClure‐Tone EB, Shiffrin ND, Pine DS, & Nelson EE (2009). Probing the Neural Correlates of Anticipated Peer Evaluation in Adolescence. Child Development, 80(4), 1000–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL, Mermelstein R, & Roesch L (2007). Sex Differences in Adolescent Depression: Stress Exposure and Reactivity Models. Child Development, 78(1), 279–295. [DOI] [PubMed] [Google Scholar]
- Hu L, & Bentler PM (1999). Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling: A Multidisciplinary Journal, 6(1), 1–55. [Google Scholar]
- Karr JE, Areshenkoff CN, Rast P, Hofer SM, Iverson GL, & Garcia-Barrera MA (2018). The unity and diversity of executive functions: A systematic review and re-analysis of latent variable studies. Psychological Bulletin, 144(11), 1147–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klavir O, Prigge M, Sarel A, Paz R, & Yizhar O (2017). Manipulating fear associations via optogenetic modulation of amygdala inputs to prefrontal cortex. Nature Neuroscience, 20(6), 836–844. [DOI] [PubMed] [Google Scholar]
- Knutson B, Westdorp A, Kaiser E, & Hommer D (2000). FMRI Visualization of Brain Activity during a Monetary Incentive Delay Task. NeuroImage, 12(1), 20–27. [DOI] [PubMed] [Google Scholar]
- Kozak MJ, & Cuthbert BN (2016). The NIMH Research Domain Criteria Initiative: Background, Issues, and Pragmatics. Psychophysiology, 53(3), 286–297. [DOI] [PubMed] [Google Scholar]
- Kozak MJ, & Miller GA (1982). Hypothetical constructs versus intervening variables: A re-appraisal of the three-systems model of anxiety assessment. Behavioral Assessment, 4(3), 347–358. [Google Scholar]
- Kraemer HC, Measelle JR, Ablow JC, Essex MJ, Boyce WT, & Kupfer DJ (2003). A new approach to integrating data from multiple informants in psychiatric assessment and research: mixing and matching contexts and perspectives. American Journal of Psychiatry, 160(9), 1566–1577. [DOI] [PubMed] [Google Scholar]
- Kramer MD, Patrick CJ, Krueger RF, & Gasperi M (2012). Delineating physiologic defensive reactivity in the domain of self-report: Phenotypic and etiologic structure of dispositional fear. Psychological Medicine, 42(6), 1305–1320. [DOI] [PubMed] [Google Scholar]
- Kupferberg A, Bicks L, & Hasler G (2016). Social functioning in major depressive disorder. Neuroscience & Biobehavioral Reviews, 69, 313–332. [DOI] [PubMed] [Google Scholar]
- Ladouceur CD, Jones NP, Hutchinson EA, Sequeira SL, Silk JS. (in preparation) Reduced activation in neural regions within an affective salience network during social reward anticipation predicts increases in symptoms of anxiety in at-risk adolescent girls.
- Lang PJ, Davis M, & Öhman A (2000). Fear and anxiety: Animal models and human cognitive psychophysiology. Journal of Affective Disorders, 61(3), 137–159. [DOI] [PubMed] [Google Scholar]
- Liebowitz MR (1987). Social phobia. Modern Problems in Pharmacopsychiatry, 22, 141–173. [DOI] [PubMed] [Google Scholar]
- Luna B, Padmanabhan A, & O’Hearn K (2010). What has fMRI told us about the development of cognitive control through adolescence?. Brain and Cognition, 72(1), 101–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLeod C, Mathews A, & Tata P (1986). Attentional bias in emotional disorders. Journal of Abnormal Psychology, 95(1), 15–20. [DOI] [PubMed] [Google Scholar]
- MacNamara A, & Phan KL (2016). Psychobiological operationalization of RDoC constructs: Methodological and conceptual opportunities and challenges. Psychophysiology, 53(3), 406–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten CL, Eisenberger NI, Borofsky LA, Pfeifer JH, McNealy K, Mazziotta JC, & Dapretto M (2009). Neural correlates of social exclusion during adolescence: Understanding the distress of peer rejection. Social Cognitive and Affective Neuroscience, 4(2), 143–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masten CL, Telzer EH, Fuligni AJ, Lieberman MD, & Eisenberger NI (2012). Time spent with friends in adolescence relates to less neural sensitivity to later peer rejection. Social Cognitive and Affective Neuroscience, 7(1), 106–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazaika PK, Whitfield S, & Cooper JC (n.d.). Detection and Repair of Transient Artifacts in fMRI Data. 1. [Google Scholar]
- McDonald AJ (1998). Cortical pathways to the mammalian amygdala. Progress in Neurobiology, 55(3), 257–332. [DOI] [PubMed] [Google Scholar]
- Merikangas KR, He JP, Burstein M, Swanson SA, Avenevoli S, Cui L, Benjet C, Georgiades K, & Swendsen J (2010). Lifetime prevalence of mental disorders in US adolescents: results from the National Comorbidity Survey Replication–Adolescent Supplement (NCS-A). Journal of the American Academy of Child & Adolescent Psychiatry, 49(10), 980–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GA, & Rockstroh B (2013). Endophenotypes in Psychopathology Research: Where Do We Stand? Annual Review of Clinical Psychology, 9(1), 177–213. [DOI] [PubMed] [Google Scholar]
- Monk CS, Klein RG, Telzer EH, Schroth EA, Mannuzza S, Moulton JL, … & Ernst M (2008). Amygdala and Nucleus Accumbens Activation to Emotional Facial Expressions in Children and Adolescents at Risk for Major Depression. American Journal of Psychiatry, 165(1), 90–98. [DOI] [PubMed] [Google Scholar]
- Moser JS, Durbin CE, Patrick CJ, & Schmidt NB (2015). Combining Neural and Behavioral Indicators in the Assessment of Internalizing Psychopathology in Children and Adolescents. Journal of Clinical Child & Adolescent Psychology, 44(2), 329–340. [DOI] [PubMed] [Google Scholar]
- Oppenheimer CW, Silk JS, Lee KH, Dahl RE, Forbes E, Ryan N, & Ladouceur CD (2019). Suicidal Ideation Among Anxious Youth: A Preliminary Investigation of the Role of Neural Processing of Social Rejection in Interaction with Real World Negative Social Experiences. Child Psychiatry and Human Development. [DOI] [PMC free article] [PubMed]
- Patrick CJ, Venables NC, Yancey JR, Hicks BM, Nelson LD, & Kramer MD (2013). A construct-network approach to bridging diagnostic and physiological domains: Application to assessment of externalizing psychopathology. Journal of Abnormal Psychology, 122(3), 902–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Edgar K, Bar-Haim Y, McDermott JM, Chronis-Tuscano A, Pine DS, & Fox NA (2010). Attention biases to threat and behavioral inhibition in early childhood shape adolescent social withdrawal. Emotion, 10(3), 349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Edgar K, Roberson-Nay R, Hardin MG, Poeth K, Guyer AE, Nelson EE, McClure EB, Henderson HA, Fox NA, Pine DS, & Ernst M (2007). Attention alters neural responses to evocative faces in behaviorally inhibited adolescents. NeuroImage, 35(4), 1538–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, & Boxer A (1988). A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence, 17(2), 117–133. [DOI] [PubMed] [Google Scholar]
- Price RB, Rosen D, Siegle GJ, Ladouceur CD, Tang K, Allen KB, Ryan ND, Dahl RE, Forbes EE, & Silk JS (2016a). From anxious youth to depressed adolescents: Prospective prediction of 2-year depression symptoms via attentional bias measures. Journal of Abnormal Psychology, 125(2), 267–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RB, Allen KB, Silk JS, Ladouceur CD, Ryan ND, Dahl RE, Forbes EE, & Siegle GJ (2016b). Vigilance in the laboratory predicts avoidance in the real world: A dimensional analysis of neural, behavioral, and ecological momentary data in anxious youth. Developmental Cognitive Neuroscience, 19, 128–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price RB, Siegle GJ, Silk JS, Ladouceur C, McFarland A, Dahl RE, & Ryan ND (2013). Sustained Neural Alterations in Anxious Youth Performing an Attentional Bias Task: A Pupilometry Study. Depression and Anxiety, 30(1), 22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby K, & Slee PT (1993). Dimensions of interpersonal relation among Australian children and implications for psychological well-being. The Journal of Social Psychology, 133(1), 33–42. [DOI] [PubMed] [Google Scholar]
- Rosen D, Price RB, & Silk JS (2019). An integrative review of the vigilance-avoidance model in pediatric anxiety disorders: Are we looking in the wrong place?. Journal of Anxiety Disorders, 64, 79–89. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, & Bates JE (2006). Temperament. In Handbook of child psychology: Social, emotional, and personality development, Vol. 3, 6th ed (pp. 99–166). John Wiley & Sons Inc. [Google Scholar]
- Roy AK, Shehzad Z, Margulies DS, Kelly AC, Uddin LQ, Gotimer K, Biswal BB, Castellanos FX, & Milham MP (2009). Functional connectivity of the human amygdala using resting state fMRI. Neuroimage, 45(2), 614–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudolph KD, & Asher SR (2000). Adaptation and maladaptation in the peer system: Developmental processes and outcomes. In Handbook of developmental psychopathology, 2nd ed (pp. 157–175). Kluwer Academic Publishers. [Google Scholar]
- Rudolph KD, & Flynn M (2014). Depression in adolescents. In Handbook of depression, 3rd ed (pp. 391–409). Guilford Press. [Google Scholar]
- Rusby JC, Westling E, Crowley R, & Light JM (2013). Concurrent and predictive associations between early adolescent perceptions of peer affiliates and mood states collected in real time via ecological momentary assessment methodology. Psychological Assessment, 25(1), 47–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P, Faber EL, Lopez de Armentia M, & Power JMJPR (2003). The amygdaloid complex: anatomy and physiology. Physiological Reviews, 83(3), 803–834. [DOI] [PubMed] [Google Scholar]
- Shirtcliff EA, Dahl RE, & Pollak SD (2009). Pubertal Development: Correspondence Between Hormonal and Physical Development. Child Development, 80(2), 327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JS, Davis S, McMakin DL, Dahl RE, & Forbes EE (2012a). Why do anxious children become depressed teenagers? The role of social evaluative threat and reward processing. Psychological Medicine, 42(10), 2095–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JS, Forbes EE, Whalen DJ, Jakubcak JL, Thompson WK, Ryan ND, Axelson DA, Birmaher B, & Dahl RE (2011). Daily emotional dynamics in depressed youth: A cell phone ecological momentary assessment study. Journal of Experimental Child Psychology, 110(2), 241–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JS, Siegle GJ, Lee KH, Nelson EE, Stroud LR, & Dahl RE (2014). Increased neural response to peer rejection associated with adolescent depression and pubertal development. Social Cognitive and Affective Neuroscience, 9(11), 1798–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk JS, Stroud LR, Siegle GJ, Dahl RE, Lee KH, Nelson EE (2012b). Peer acceptance and rejection through the eyes of youth: pupillary, eyetracking and ecological data from the Chatroom Interact task. Social Cognitive and Affective Neuroscience, 7, 93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spithoven AWM, Bijttebier P, & Goossens L (2017). It is all in their mind: A review on information processing bias in lonely individuals. Clinical Psychology Review, 58, 97–114. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, Marcus DJ, Westerlund A, Casey B, & Nelson C (2009). The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research, 168(3), 242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venables NC, Yancey JR, Kramer MD, Hicks BM, Krueger RF, Iacono WG, Joiner TE, & Patrick CJ (2018). Psychoneurometric assessment of dispositional liabilities for suicidal behavior: Phenotypic and etiological associations. Psychological Medicine, 48(3), 463–472. [DOI] [PubMed] [Google Scholar]
- Wellman LL, Forcelli PA, Aguilar BL, & Malkova L (2016). Bidirectional control of social behavior by activity within basolateral and central amygdala of primates. Journal of Neuroscience, 36(33), 8746–8756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey JR, Venables NC, & Patrick CJ (2016). Psychoneurometric operationalization of threat sensitivity: Relations with clinical symptom and physiological response criteria. Psychophysiology, 53(3), 393–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


