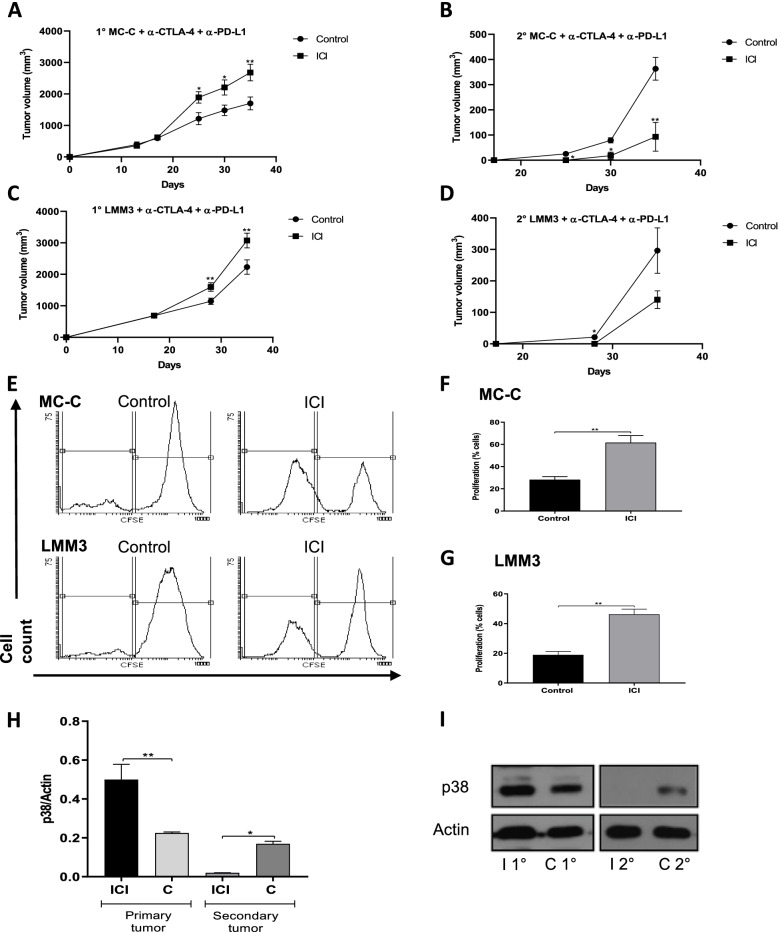
Fig. 4.
Simultaneous effects of immune checkpoint inhibitors (ICI) on primary large-sized tumors and secondary tumor-bearing mice. (A, B, C, D) Mice bearing MC-C (A) or LMM3 tumor (C) measuring 800 mm3 (1°MC-C or 1°LMM3, respectively) were challenged at day 17 with a secondary tumor implant (B, D) carried out in the contralateral flank (2°MC-C or 2°LMM3, respectively) and, simultaneously, treated with ICI (anti-CTLA-4 + anti-PD-L1). 5 × 105 tumor cells were inoculated for MC-C and LMM3 primary tumors, and 2 × 105 tumor cells for secondary implants. For each tumor, the figure shows a representative experiment (n = 4–6 mice per group) out of two experiments that rendered similar results. Data were expressed as mean (mm3) ± SEM of tumor volume. (E) Representative CFSE flow cytometric histograms of splenic T-cells from MC-C and LMM3 primary and secondary tumors bearing mice. (F, G) Percentage of the proliferation of splenic T cells from MC-C (F) and LMM3 (G) primary and secondary tumors bearing mice. Mice were treated with anti-CTLA-4 + anti-PD-L1 (immunized group), and non-treated mice served as control (control group). Each value represents the mean ± SEM of two assays. Each dose of anti-CTLA-4 and anti-PD-L1 was 100 μg per mouse. For simplicity, groups treated with anti-CTLA-4 alone and anti-PD-L1 alone were omitted. (H, I) Expression of phosphorylated (p)-38 (p38) by Western blotting. Macrophages (3 × 106 cells) were collected surrounding the s.c. primary and secondary MC-C tumors 7 days post-secondary implant. Mice were treated with anti-CTLA-4 + anti-PD-L1 (immunized group), and non-treated mice served as control. A representative experiment is shown. The figure shows levels of p38 in the different groups, normalized with beta-actin densitometric units, representing the mean ± SE of three independent experiments. Statistical comparison between experimental groups and control: * p < 0.02; ** p < 0.01; *** p < 0.001. Immunized primary tumor (I 1°), immunized secondary tumor (I 2°), control primary tumor (C 2°) and control secondary tumor (C 2°).