Figure 5. ATCs armed with hu3F8-BsAb suppressed growth of NB cell line and patient derived xenografts.
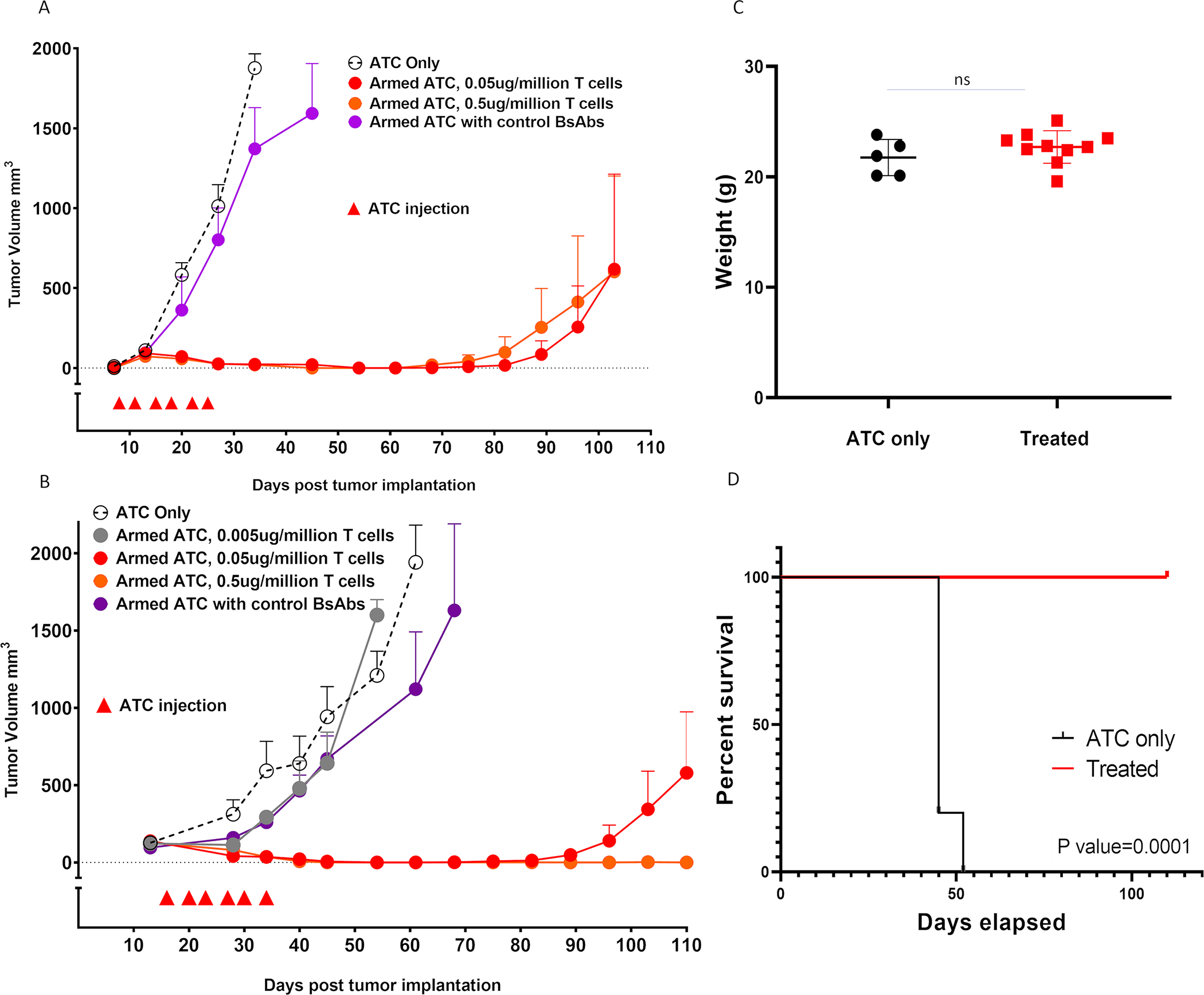
(A) Anti-tumor response among IMR32 xenografts (n=5 mice/group), (B) Anti-tumor response among patient derived xenografts (n=5 mice/group), (C) Body weight during the first 50 days of treatment in B, and (D) Improvement of survival after armed ATC treatment in B. All treatments were given biweekly for total 6 times, with 20 million armed ATCs. Control BsAbs were given at BsAb concentration of 0.05 ug/million T cells.
