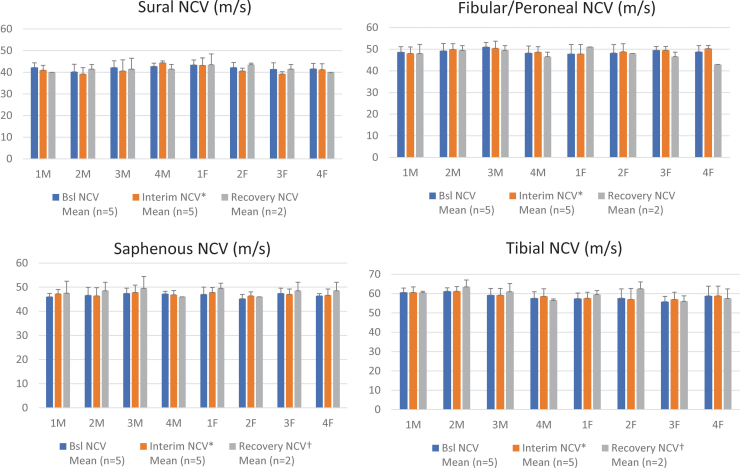
Figure 5.
Neuroelectrophysiologic evaluations. (A) Sural (sensory) nerve conduction, (B) fibular/peroneal (sensory) nerve conduction, (C) saphenous (sensory) nerve conduction, and (D) tibial (motor) nerve conduction in males (M) and females (F) was determined at baseline, 2 weeks postdose (interim) and 13 weeks postdose (recovery) for NHPs in all four groups treated with onasemnogene abeparvovec. Group 1 was administered vehicle control item and contrast agent only. Group 2 was administered onasemnogene abeparvovec (3 × 1013 vg/animal) without immunosuppression. Group 3 was administered onasemnogene abeparvovec (3 × 1013 vg/animal) along with oral prednisolone (1 mg/kg). Group 4 was administered onasemnogene abeparvovec (3 × 1013 vg/animal) and a combination of intravenous rituximab and oral everolimus. NCV are all L (m/s). NCV, nerve conduction velocity.