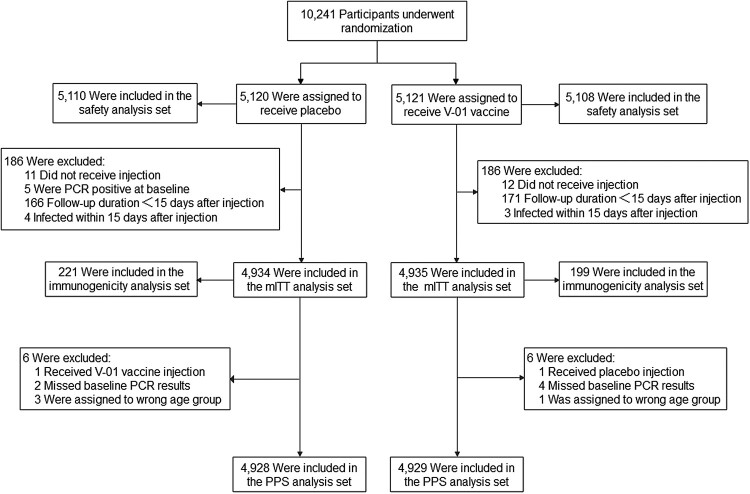
Figure 1.
Summary of participants. The primary efficacy analysis was performed based on the modified intent-to-treat (mITT) set. A total of 10,241 eligible participants were randomly assigned to receive V-01 vaccine or placebo. After a 2-month follow-up, 4,935 participants in the V-01 vaccine group and 4,934 in the placebo group were included in the MITT. Of these, 199 and 221 participants in V-01 vaccine and placebo group, respectively, formed the immunogenicity analysis set, blood samples were obtained at baseline and at days 14 and 28 to assess the anti-SARS-CoV-2 neutralizing antibody titres.