Abstract
Background
Coronavirus disease 2019 (COVID‐19) is now the third leading cause of death in the United States. Malnutrition in hospitalized patients increases risk of complications. However, the effect of malnutrition on outcomes in patients infected is unclear. This study aims to identify the impact of malnutrition on mortality and adverse hospital events in patients hospitalized with COVID‐19.
Methods
This study used data from the National COVID Cohort Collaborative (N3C), a COVID‐19 repository containing harmonized, longitudinal electronic health record data from US health systems. Malnutrition was categorized into three groups based on condition diagnosis: (1) none documented, (2) history of malnutrition, and (3) hospital‐acquired malnutrition. Multivariable logistic regression was performed to determine whether malnutrition was associated with mortality and adverse events, including mechanical ventilation, acute respiratory distress syndrome, extracorporeal membrane oxygenation, and hospital‐acquired pressure injury, in hospitalized patients with COVID‐19.
Results
Of 343,188 patients hospitalized with COVID‐19, 11,206 had a history of malnutrition and 15,711 had hospital‐acquired malnutrition. After adjustment for potential confounders, odds of mortality were significantly higher in patients with a history of malnutrition (odds ratio [OR], 1.71; 95% confidence interval [CI], 1.63–1.79; P < 0.001) and hospital‐acquired malnutrition (OR, 2.5; 95% CI, 2.4–2.6; P < 0.001). Adjusted odds of adverse hospital events were also significantly elevated in both malnutrition groups.
Conclusions
Results indicate the risk of mortality and adverse inpatient events in adults with COVID‐19 is significantly higher in patients with malnutrition. Prevention, diagnosis, and treatment of malnutrition could be a key component in improving outcomes in these patients.
Keywords: adult, malnutrition, nutrition assessment, nutrition support practice, outcomes research/quality, pulmonary disease
CLINICAL RELEVANCY STATEMENT
The coronavirus disease 2019 (COVID‐19) pandemic has become a significant cause of morbidity and mortality worldwide. Nutrition status has been shown to impact morbidity and mortality; therefore, the purpose of this study was to identify the impact of malnutrition on clinical outcomes in patients hospitalized with COVID‐19. In this cohort study of 343,188 patients with COVID‐19 from 59 sites across the United States, those with malnutrition had higher unadjusted and adjusted risks for mortality and adverse hospital events. These findings suggest that diagnosis and interventions for malnutrition could play a role in improving outcomes in patients hospitalized with COVID‐19.
INTRODUCTION
The coronavirus disease 2019 (COVID‐19) pandemic has swept the globe, with >500 million cases and >6 million deaths worldwide. Several factors, including advanced age, 1 male sex, 1 obesity, 2 smoking, 3 and the number of comorbidities, 4 have been shown to increase mortality in patients diagnosed with COVID‐19. Nutrition status is often significantly impacted in individuals with one or more of these factors because of suboptimal dietary intake or altered nutrient metabolism. 5 , 6 , 7 , 8 , 9 As malnutrition is associated with weaker immune responses 10 and poor diaphragmatic and respiratory muscle function, 11 it is likely that individuals with preexisting malnutrition may be more susceptible to poor outcomes during severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) infection. Conversely, those without underlying malnutrition may become acutely malnourished during admission, owing to symptoms of COVID‐19 such as anorexia, nausea, vomiting, and hypermetabolism. 12 Whereas previous studies have explored the relationship between nutrition risk and malnutrition in patients hospitalized with COVID‐19, the impact of acute and chronic malnutrition on clinical outcomes has not been reported in a large US cohort.
Malnutrition in hospitalized patients has been associated with poor outcomes, including increased risk of infection, increased length of stay (LOS), and higher in‐hospital mortality rates. 13 Furthermore, malnutrition is significantly associated with lung function and is an independent risk factor for acute respiratory distress syndrome (ARDS). 14 , 15 Therefore, we hypothesized that malnourished patients hospitalized with COVID‐19 have poor clinical outcomes and that clinical outcomes may vary on the basis of malnutrition onset. Previous research conducted outside the United States has extended our understanding of the impact of malnutrition on mortality and LOS in patients hospitalized with COVID‐19. In a systematic review of 14 articles published in China and Europe between January and May 2020, the odds of mortality increased by 10 times in patients with malnutrition, as defined by different types of screening and diagnostic tools. 16 A smaller study (n = 27) by Nicolau et al showed that mortality rates and intensive care unit admission were greater among patients with any degree of malnutrition than in well‐nourished patients. 17 However, several gaps in our knowledge of COVID‐19 and malnutrition remain. First, limited US data are available that assess the prevalence of malnutrition in patients hospitalized with COVID‐19. Second, there are no US studies assessing the effect of malnutrition on outcomes in patients hospitalized with COVID‐19. Third, differential effects of historical vs hospital‐acquired malnutrition on outcomes in patients with COVID‐19 have not been investigated.
To enable the rapid collection and analysis of clinical, laboratory, and diagnostic data from hospitals and healthcare plans, the National Institutes of Health (NIH) established the National COVID Cohort Collaborative (N3C) Data Enclave. 18 The N3C systematically collects regularly updated data derived from the electronic health record (EHR) of patients who were tested for SARS‐CoV‐2 (by polymerase chain reaction, antigen, or antibody testing) or had a diagnosis of COVID‐19 from a provider. The N3C is a secure platform through which harmonized contributing partners provide clinical data. Data partners consist of contributing institutions, encompassing multiple providers and multiple care sites, making this longitudinal data repository appropriate for researching COVID‐19. This study used the N3C database to address the impact of preexisting and hospital‐acquired malnutrition on mortality and adverse hospital events in a large US cohort.
METHODS
N3C
This retrospective cohort study uses data from 59 health centers across the United States that reported data to the N3C and includes retrospective records dating back to January 1, 2018, for historical medical context for all individuals. The Data Enclave, described in detail by Haendel et al, 18 is approved under the authority of the NIH Institutional Review Board (#IRB00249128), with Johns Hopkins University School of Medicine as the central IRB. The N3C approved a data‐use agreement completed by the University of Nebraska Medical Center, an N3C contributing partner, and a data‐use request completed by this team of investigators.
The final data extraction was completed on December 2, 2021, in the Observational Medical Outcomes Partnership (OMOP) Common Data Model (CDM) version 5.3.1. Given that reporting practices by N3C data partners vary, a data analysis plan was developed to determine minimum fact reporting per patient across key domains. Patients with missing data on sex or age and sites with limited death reporting, which was a primary outcome, were excluded (Figure 1). This follows a similar approach used by the four source data models, which all rely on data‐quality dashboards to enhance site reporting for inclusion in network studies: OMOP 19 Accrual to Clinical Trials (ACT), 20 TriNetX, 21 and Patient‐Centered Clinical Research Network (PCORnet). 22
Figure 1.
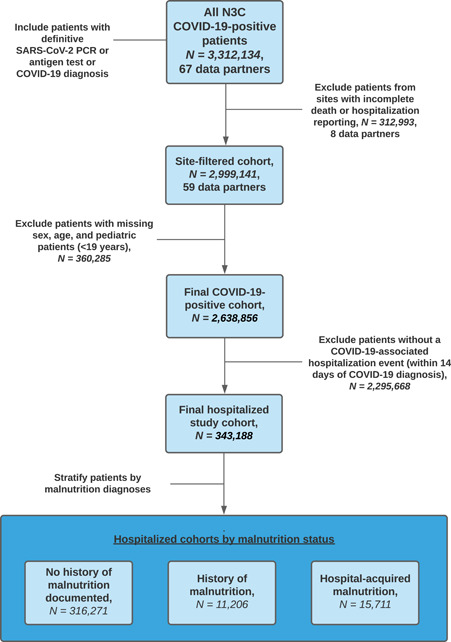
Data analysis plan. COVID‐19, coronavirus disease 2019; N3C, National COVID Cohort Collaborative; PCR, polymerase chain reaction; SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2
All clinical concept sets were created collaboratively within the N3C Enclave, with at least one informatician and one clinical subject‐matter expert reviewing each relevant concept set. An overview of the ingestion and harmonization process, sampling approaches, and overall structure of the N3C Enclave, concept set definitions, and the computable phenotypes used can be found in the Supporting Information.
Primary exposure
Malnutrition served as the primary exposure in this study, as defined by the presence of one or more of the following diagnostic codes within a patient's medical record: malnutrition, severe protein‐calorie malnutrition, malnutrition of moderate degree, moderate protein‐energy malnutrition, moderate protein‐calorie malnutrition, mild protein‐calorie malnutrition, malnutrition of mild degree, malnutrition following gastrointestinal surgery, starvation, semistarvation, undernutrition, deficiency of macronutrients, nutritional deficiency disorder, nutritional wasting, wasting disease, marasmic kwashiorkor, and kwashiorkor. Patients hospitalized within 14 days of COVID‐19 diagnosis were separated into three malnutrition groups: no history of malnutrition, history of malnutrition (malnutrition diagnosed before SARS‐CoV‐2 infection), and hospital‐acquired malnutrition (malnutrition diagnosed on or after SARS‐CoV‐2 infection). Patients with a documented history of malnutrition were included in the history of malnutrition group and not considered for the hospital‐acquired malnutrition group.
Outcomes
The primary outcome of this study was mortality, defined as death or transfer to hospice during COVID‐19 hospitalization. Secondary outcomes included the following adverse hospital events: mechanical ventilation, ARDS, extracorporeal membrane oxygenation (ECMO), and hospital‐acquired pressure injuries (HAPIs) at any time during hospitalization. Hospital visits without an end date during the study period were excluded.
Other covariates
Confounding variables included age, sex, race and ethnicity, Charlson comorbidity index (CCI), 23 and smoking status. Additional covariates include body mass index (BMI; calculated as weight [kg] divided by square height [m2]), geographic region, comorbidity incidence, LOS, and inpatient therapeutic exposure.
Statistical analysis
Frequencies and percentages of demographic and clinical characteristics between the no documented history of malnutrition, history of malnutrition, and hospital‐acquired malnutrition were calculated. Groups were then compared using either the Wilcoxon rank sum tests for continuous measures or chi‐square tests for categorical variables. Univariable and multivariable logistic regression models were used to evaluate odds ratios (ORs) of mortality and adverse hospital events (mechanical ventilation, ARDS and ECMO, and HAPIs) based on malnutrition classification. Adjusted models were controlled for age, sex, race and ethnicity, CCI, and smoking status. Confounders were determined a priori using directed acyclical graphing, knowledge of clinical relationships, and relevant literature. All statistical analyses were performed in R v3.5.1 within the N3C platform. A P value of <0.05 was considered statistically significant. All P values presented are for two‐sided tests.
A sensitivity analysis that considered differences in therapeutic exposures, covariates, and outcome timing was performed. Interaction terms between medication exposure and malnutrition status were evaluated to assess for potential differences in treatment associated with malnutrition status. In a second analysis, we assessed additional covariates, including vitals, BMI, and region. Given that covariates either are associated with adverse outcomes or vary based on data partners' reporting differences, each was excluded from the final analyses. Finally, the temporal definitions of outcomes were varied to assess the impact of time intervals on adverse events from COVID‐19 diagnosis and hospital admission.
RESULTS
A total of 343,188 patients hospitalized with COVID‐19 across the United States (Figure 2) were included in the final analysis, including 316,271 (92%) without a documented history of malnutrition, 11,206 (3.3%) with a history of malnutrition, and 15,711 (4.6%) with hospital‐acquired malnutrition (Table 1). Patients with malnutrition at any time were older than those without malnutrition (median age of 66 and 67 vs 59 years, respectively) and had a higher proportion of current or former smokers (33% and 28% vs 21%). Those with a history of malnutrition had a higher CCI (5; interquartile range [IQR], 3–8) than those without malnutrition (0; IQR, 0–2) or hospital‐acquired malnutrition (0; IQR, 0–4). Patients with hospital‐acquired malnutrition had a significantly longer LOS (13 days; IQR, 6–27) than those without malnutrition (4 days; IQR, 1–8) or a history of malnutrition (7 days; IQR, 3–14).
Figure 2.
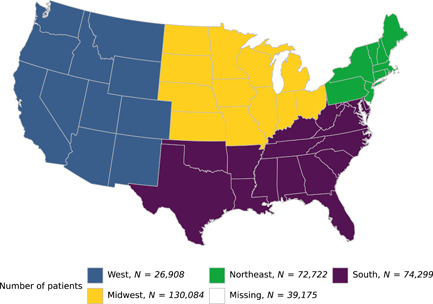
Geographic distribution of the cohort
Table 1.
Baseline characteristics of patients who were hospitalized and positive for COVID‐19, by malnutrition status
| Characteristic | No Hx of malnutrition, n = 316,271a | Hx of malnutrition, n = 11,206a | HAC malnutrition, n = 15,711a | P valueb |
|---|---|---|---|---|
| Age, years | 59 (43–71) | 66 (53–76) | 67 (56, 77) | <0.001 |
| Age group | <0.001 | |||
| <29 years | 28,389 (9.0%) | 550 (4.9%) | 417 (2.7%) | |
| 30–49 years | 77,091 (24%) | 1692 (15%) | 2065 (13%) | |
| 50–64 years | 89,476 (28%) | 3043 (27%) | 4405 (28%) | |
| >65 years | 121,315 (38%) | 5921 (53%) | 8824 (56%) | |
| Sex | <0.001 | |||
| Female | 157,830 (50%) | 5493 (49%) | 6396 (41%) | |
| Male | 158,441 (50%) | 5713 (51%) | 9315 (59%) | |
| Race and ethnicity | <0.001 | |||
| Non‐Hispanic White | 163,900 (52%) | 6288 (56%) | 8111 (52%) | |
| Black or African American | 63,337 (20%) | 2556 (23%) | 2994 (19%) | |
| Hispanic or Latino | 52,031 (16%) | 1240 (11%) | 2341 (15%) | |
| Other | 28,751 (9.1%) | 891 (8.0%) | 1682 (11%) | |
| Missing/unknown | 8252 (2.6%) | 231 (2.1%) | 583 (3.7%) | |
| BMI | 30 (26–36) | 25 (21–30) | 28 (23–33) | <0.001 |
| BMI category | <0.001 | |||
| <18.5 | 4183 (1.3%) | 979 (8.7%) | 682 (4.3%) | |
| 18.5–24.9 | 35,558 (11%) | 3924 (35%) | 3864 (25%) | |
| 25–29.9 | 54,631 (17%) | 2289 (20%) | 3776 (24%) | |
| ≥30 | 112,175 (35%) | 2510 (22%) | 5248 (33%) | |
| Missing/unknown | 109,724 (35%) | 1504 (13%) | 2141 (14%) | |
| CCI | 0 (0–2) | 5 (3–8) | 1 (0–4) | <0.001 |
| CCI category | <0.001 | |||
| <1 | 182,747 (58%) | 559 (5.0%) | 7509 (48%) | |
| 1–3 | 86,979 (28%) | 2939 (26%) | 4220 (27%) | |
| >3 | 46,545 (15%) | 7708 (69%) | 3982 (25%) | |
| Region | <0.001 | |||
| Northeast | 67,233 (21%) | 2331 (21%) | 3158 (20%) | |
| South | 69,362 (22%) | 2128 (19%) | 2809 (18%) | |
| Midwest | 120,256 (38%) | 4193 (37%) | 5635 (36%) | |
| West | 24,064 (7.6%) | 1084 (9.7%) | 1760 (11%) | |
| Missing/unknown | 35,356 (11%) | 1470 (13%) | 2349 (15%) | |
| Comorbidity incidence | ||||
| Diabetes mellitus | 67,002 (21%) | 5782 (52%) | 4135 (26%) | <0.001 |
| Myocardial infarction | 13,834 (4.4%) | 2344 (21%) | 1082 (6.9%) | <0.001 |
| Congestive heart failure | 30,160 (9.5%) | 4334 (39%) | 2049 (13%) | <0.001 |
| Peripheral vascular disease | 24,418 (7.7%) | 3571 (32%) | 1897 (12%) | <0.001 |
| Stroke | 22,102 (7.0%) | 3363 (30%) | 1872 (12%) | <0.001 |
| Dementia | 8240 (2.6%) | 1574 (14%) | 976 (6.2%) | <0.001 |
| Chronic pulmonary disease | 45,757 (14%) | 4617 (41%) | 2509 (16%) | <0.001 |
| Rheumatologic disease | 10,859 (3.4%) | 1148 (10%) | 669 (4.3%) | <0.001 |
| Mild or severe liver disease | 17,300 (5.5%) | 3306 (30%) | 1339 (8.5%) | <0.001 |
| Peptic ulcer disease | 3469 (1.1%) | 1160 (10%) | 293 (1.9%) | <0.001 |
| Hemiplegia or paraplegia | 3724 (1.2%) | 967 (8.6%) | 365 (2.3%) | <0.001 |
| Renal disease | 35,075 (11%) | 4820 (43%) | 2711 (17%) | <0.001 |
| Any malignancy (except skin) | 22,670 (7.2%) | 3348 (30%) | 2142 (14%) | <0.001 |
| Metastatic solid tumor | 4348 (1.4%) | 1277 (11%) | 610 (3.9%) | <0.001 |
| HIV/AIDS | 1734 (0.5%) | 216 (1.9%) | 125 (0.8%) | <0.001 |
| Current or former smoker | 66,863 (21%) | 3675 (33%) | 4351 (28%) | <0.001 |
| Outcomes | ||||
| Death or transfer to hospice | 33,831 (11%) | 2930 (26%) | 4525 (29%) | <0.001 |
| Invasive mechanical ventilation | 22,211 (7.0%) | 1402 (13%) | 5068 (32%) | <0.001 |
| ARDS | 116,992 (37%) | 5011 (45%) | 9926 (63%) | <0.001 |
| ECMO | 1235 (0.4%) | 78 (0.7%) | 533 (3.4%) | <0.001 |
| Hospital‐acquired pressure injury | 5894 (1.9%) | 1083 (9.7%) | 2216 (14%) | <0.001 |
| Length of stay | 4 (1, 8) | 7 (3, 14) | 13 (6, 27) | <0.001 |
| Inpatient therapeutic exposure | ||||
| Remdesivir | 53,143 (17%) | 1977 (18%) | 4575 (29%) | <0.001 |
| Steroid | 61,717 (20%) | 2794 (25%) | 5103 (32%) | <0.001 |
| Vasopressor | 17,521 (5.5%) | 1184 (11%) | 3052 (19%) | <0.001 |
Abbreviations: ARDS, acute respiratory distress syndrome; BMI, body mass index; CCI, Charlson comorbidity index; ECMO, extracorporeal membrane oxygenation; HAC, xxx; Hx, history.
Statistics presented: median (interquartile range) or n (%).
Statistical tests performed: Wilcoxon rank sum test; chi‐square test of independence.
Malnutrition and mortality during COVID‐19 hospitalization
In the unadjusted analysis, odds of mortality were significantly higher in patients with a history of malnutrition (OR, 2.96; 95% confidence interval [CI], 2.83–3.09; P < 0.001) and hospital‐acquired malnutrition (OR, 3.38; 95% CI, 3.26–3.5; P < 0.001) (Table 2; Figure 3A). After adjustment for age, smoking status, race, and CCI, the results remained significant, as odds of mortality were 1.7 times higher in patients with a history of malnutrition (adjusted OR [aOR], 1.71; 95% CI, 1.63–1.79; P < 0.001) and 2.5 times higher in those with hospital‐acquired malnutrition (aOR, 2.5; 95% CI, 2.4–2.6; P < 0.001) (Table 3; Figure 3B).
Table 2.
Adjusted odds ratios for adverse events by malnutrition status in the National COVID Cohort Collaborative
| Nutrition deficiency status | Death or transfer to hospicea | Invasive mechanical ventilationa | Acute respiratory distress syndromea | Extracorporeal membrane oxygenationa | Hospital‐acquired pressure injurya |
|---|---|---|---|---|---|
| No documented Hx of malnutrition (ref) | — | — | — | — | — |
| History of malnutrition | 2.96 (2.83–3.09) | 1.89 (1.79–2.00) | 1.38 (1.33–1.43) | 1.79 (1.41–2.23) | 5.63 (5.26–6.03) |
| Hospital‐acquired malnutrition | 3.38 (3.26–3.50) | 6.30 (6.08–6.54) | 2.92 (2.83–3.02) | 8.96 (8.08–9.92) | 8.65 (8.21–9.11) |
Abbreviations: —, not applicable; Hx, history; ref, reference.
Adjusted odds ratio (95% confidence interval).
Figure 3.
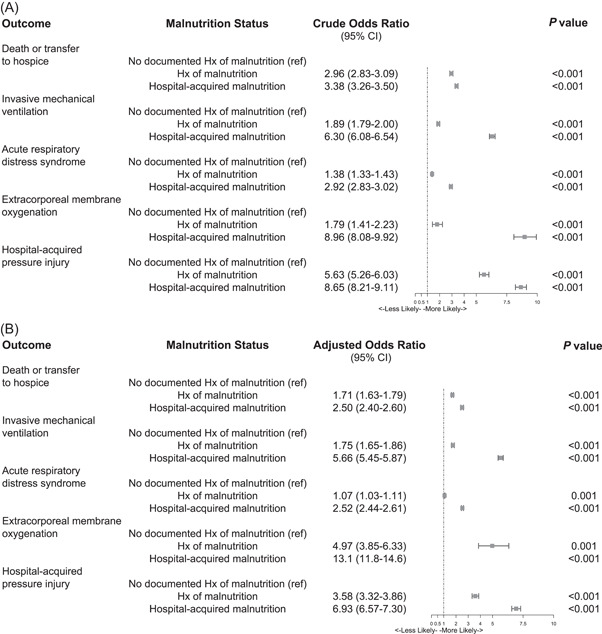
Forest plots showing the crude and adjusted odds ratios for inpatient mortality or transfer to hospice by malnutrition status. (A) Crude odds ratios of adverse event by malnutrition status of individuals infected with severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2). (B) Adjusted odds ratios of adverse event by malnutrition status of individuals infected with SARS‐CoV‐2. CI, confidence interval; HAC, xxx; Hx, history; ref, reference
Table 3.
Forrest plots of crude and adjusted odds ratios of adverse event by malnutrition status in SARS‐CoV‐2 Infected Persons
| Variable | Death or transfer to hospicea | Invasive mechanical ventilationa | Acute respiratory distress syndromea | Extracorporeal membrane oxygenationa | Hospital‐acquired pressure injurya |
|---|---|---|---|---|---|
| Nutrition deficiency status | |||||
| No documented Hx of malnutrition (ref) | — | — | — | — | — |
| History of malnutrition | 1.71 (1.63–1.79) | 1.75 (1.65–1.86) | 1.07 (1.03–1.11) | 4.97 (3.85–6.33) | 3.58 (3.32–3.86) |
| Hospital‐acquired malnutrition | 2.50 (2.40–2.60) | 5.66 (5.45–5.87) | 2.52 (2.44–2.61) | 13.1 (11.8–14.6) | 6.93 (6.57–7.30) |
| Age | 1.05 (1.05–1.05) | 1.01 (1.01–1.01) | 1.01 (1.01–1.02) | 0.96 (0.96–0.97) | 1.02 (1.02–1.02) |
| Sex | |||||
| Female (ref) | — | — | — | — | — |
| Male | 1.40 (1.37–1.43) | 1.57 (1.53–1.61) | 1.26 (1.24–1.27) | 2.20 (1.99–2.44) | 1.29 (1.24–1.35) |
| Race and ethnicity | |||||
| Non‐Hispanic White (ref) | — | — | — | — | — |
| Black or African American | 0.95 (0.93–0.98) | 1.21 (1.17–1.25) | 1.06 (1.04–1.08) | 0.81 (0.70–0.93) | 1.06 (1.00–1.12) |
| Hispanic or Latino | 1.06 (1.02–1.09) | 1.34 (1.29–1.39) | 1.25 (1.22–1.27) | 1.32 (1.17–1.49) | 0.97 (0.91–1.04) |
| Missing/unknown | 1.35 (1.26–1.45) | 1.85 (1.73–1.98) | 1.36 (1.31–1.43) | 1.94 (1.58–2.37) | 1.31 (1.15–1.48) |
| Other | 1.06 (1.02–1.10) | 1.17 (1.12–1.22) | 0.95 (0.92–0.97) | 1.45 (1.25–1.68) | 1.29 (1.21–1.39) |
| Charlson comorbidity index | 1.10 (1.09–1.10) | 1.00 (1.00–1.01) | 1.04 (1.04–1.05) | 0.80 (0.77–0.83) | 1.08 (1.07–1.09) |
| Smoking status | |||||
| Nonsmoker (ref) | — | — | — | — | — |
| Current or former smoker | 1.03 (1.00–1.06) | 1.24 (1.21–1.28) | 1.04 (1.02–1.06) | 0.70 (0.62–0.79) | 1.04 (0.99–1.10) |
Abbreviations: —, not applicable; Hx, history; ref, reference.
Adjusted odds ratio (95% confidence interval).
Adverse hospital events during COVID‐19 hospitalization
Adverse hospital events of patients with COVID‐19 were significantly associated with malnutrition in the univariable analysis (Table 2; Figure 3A). Fully adjusted models show increased odds of mechanical ventilation in patients with a history of malnutrition (aOR, 1.75; 95% CI, 1.65–1.86; P < 0.001) or hospital‐acquired malnutrition (aOR, 5.66; 95% CI, 5.45–5.87; P < 0.001). Odds of developing ARDS or requiring ECMO increased by 7.0% (aOR, 1.07; 95% CI, 1.03–1.11; P = 0.001) and 4.97 times (aOR, 4.97; 95% CI, 3.85–6.33; P < 0.001), respectively, in patients with a history of malnutrition. Those with hospital‐acquired malnutrition had significantly higher odds of developing ARDS (aOR, 2.52; 95% CI, 2.44–2.61; P < 0.001) and requiring ECMO (aOR, 13.1; 95% CI, 11.8–14.6; P < 0.001). Odds of developing a HAPI were 5.6 times higher in those with a history of malnutrition (aOR, 5.63; 95% CI, 5.26–6.03; P < 0.001) and 8.7 times higher in patients with hospital‐acquired malnutrition (aOR, 8.65; 95% CI, 8.21–9.11; P < 0.001) (Table 3; Figure 3B).
Sensitivity analyses
Results from sensitivity analyses—including therapeutic exposures, therapy by malnutrition interaction terms, inclusion of additional covariates (BMI, region, vitals), and differences in outcome definitions—yielded findings similar to those reported. Because of differences in reporting standards and their role in the causal pathway, these covariates were excluded. These sensitivity analyses did not impact the interpretation of results.
DISCUSSION
This large retrospective study of patients with COVID‐19 who had extensive clinical information showed that a diagnosis of malnutrition, either before COVID‐19 diagnosis or during hospitalization for COVID‐19, significantly increased the odds of mortality and adverse events during admission. To our knowledge, this is the first multisite study with patients sampled from across the United States to evaluate the impact of malnutrition on mortality in patients hospitalized with COVID‐19 and to elucidate variances in outcomes between patients with COVID‐19 and malnutrition before infection and patients who develop malnutrition at or after diagnosis. These findings highlight the need for careful nutrition assessment, intervention, and management of the patient population with COVID‐19.
Adequate nutrition plays a significant role in immune function by inhibiting immune cell activation and maintaining intestinal barrier integrity. Malnutrition has been considered a leading cause of immunodeficiency worldwide, leaving those affected more susceptible to infection. 24 Evidence suggests population‐level malnutrition may be related to fatal COVID‐19 infections. 25 Thomas et al investigated the metabolic effects of COVID‐19 by analyzing serum metabolites from patients with COVID‐19 in comparison with negative controls and found an increase of free fatty acids in circulation, especially in those with high levels of inflammatory cytokines. 26 Significant alterations in nitrogen and carbon metabolism may be due to the significant and prolonged, progressive hypermetabolism described in this population. 27 In addition, the cytokine storm generated by the host in response to infection, resulting in an extreme inflammation process, has been shown to induce loss of skeletal muscle in respiratory diseases. 28
Our findings build upon smaller studies, conducted mostly in Asia and Europe, describing the impact of malnutrition on patients hospitalized with COVID‐19. Previous research has shown increased mortality in this patient population, but results have been limited by small sample sizes, 17 , 29 , 30 , 31 , 32 single‐site studies, 17 , 29 , 30 specific patient populations, 30 , 33 and short enrollment periods during the first wave of the pandemic. 29 , 34 , 35 Of studies reporting a significant impact of malnutrition on mortality, 31 , 32 , 33 , 34 , 35 each determined nutrition status on the basis of a nutrition risk screen, which may not have allowed researchers to identify the risk of mortality in patients with chronic malnutrition not caused by COVID‐19 and those who developed malnutrition during COVID‐19 hospitalization.
Our study found malnutrition was associated with increased risks for adverse hospital events, including mechanical ventilation, ARDS, ECMO, and HAPI. The impact of poor nutrition status in hospitalized patients has been demonstrated in other viral respiratory diseases, including a study of a large‐scale influenza outbreak in Japan. Predictors of mortality and infection upon hospital admission from influenza in 579 patients included malnutrition. Adjusted models showed malnutrition was significantly associated with 30‐day survival from influenza onset (hazard ratio, 3.1; 95% CI, 1.35–7.26; P = 0.008) and pneumonia on admission (OR, 2.5; 95% CI, 1.28–4.94; P = 0.007). 36 We also found an increased risk of HAPI in patients hospitalized with COVID‐19, which has been associated in other populations such as long‐term care residents. In a study conducted in Australia, odds of pressure ulcer presence were 1.9 times higher (aOR, 1.9; 95% CI, 1.3–2.7; P < 0.001) in long‐term care residents with malnutrition than in those who were well nourished. 37
The following limitations to this research are recognized. During the initial stages of the pandemic, the overwhelmed healthcare system may have limited opportunities for in‐depth nutrition assessment, which may have contributed to an inability to consistently characterize malnutrition. These data are retrospective and used International Statistical Classification of Diseases, Tenth Revision (ICD‐10) diagnostic codes, which are highly reliant on provider documentation of malnutrition and may vary considerably among providers. Furthermore, patients with a history of malnutrition were included in the history of malnutrition group and not considered for the no history or hospital‐acquired malnutrition group. Because of the restrictions of working with discreet data fields, we are unable to identify persons with resolved, exacerbated, or worsened malnutrition, which would only be possible with chart review. The standard of care would be to identify characteristics recommended for the identification and documentation of adult malnutrition based on the Academy of Nutrition and Dietetics (AND) and American Society for Parenteral and Enteral Nutrition (ASPEN) guidelines. 38 According to these guidelines, two or more of the following characteristics are recommended for diagnosis: insufficient energy intake, weight loss, loss of muscle mass, loss of subcutaneous fat, localized or generalized fluid accumulation, and diminished functional status as measured by handgrip strength. Use of the AND/ASPEN guidelines for diagnosis of malnutrition is important, as identifying muscle wasting may be crucial in identifying populations at the highest risk for morbidity and mortality. 39 It is also possible that our results were due to other specific micronutrient deficiencies. For example, in a South Korean population, selenium and vitamin D deficiencies were highly prevalent in patients with COVID‐19. Vitamin D metabolites have long been known to support antiviral mechanisms, including induction of antimicrobial peptides, and vitamin D supplementation has been associated with reduced concentration of markers of systemic inflammation. 40 We also noted a discrepancy in severity of outcomes between the groups with a history of malnutrition and those with hospital‐acquired malnutrition. It is possible that those with a history of malnutrition were more likely to have less inflammation present, consistent with malnutrition of chronic disease‐related malnutrition with a mild to moderate degree of inflammation.
In contrast, those patients diagnosed with malnutrition during their admission for COVID‐19 could have had malnutrition related to an acute disease with a marked inflammatory response present. These overlapping insults may explain the higher risks of adverse events in the group with hospital‐acquired malnutrition. 13 N3C contains EHR data from sites with diverse patient populations and differences in data reporting that may result in misclassification of comorbid conditions and malnutrition reporting based on degree of hospital interaction in the period leading up to COVID‐19 hospitalization. We report a similar comorbid burden as other national studies using more homogenous EHR‐based data sources. 41 Still, malnutrition relies on provider identification, which is known to be universally low across most care settings. 42 Although we anticipate nondifferential misclassification and likely a significant underestimation of the impact of malnutrition due to underreporting, we acknowledge that all comparisons are made with patients lacking documented malnutrition rather than reflecting a true absence of malnutrition.
This study highlights the need for screening and assessment processes that identify malnutrition early, with interventions to attenuate the effects of malnutrition. It appears likely COVID‐19 will not be eradicated. As we continue to learn how to manage this syndrome, it becomes imperative to ensure nutrition needs are met. Prevention, diagnosis, and treatment of malnutrition could be a key component in improving outcomes in patients hospitalized with COVID‐19.
AUTHOR CONTRIBUTIONS
Jana Ponce, Megan Timmerman, Alfred Jerrod Anzalone, and Corrine Hanson contributed to conception/design of the research; Jana Ponce, Megan Timmerman, Alfred Jerrod Anzalone, Kristina Bailey, Harlan Sayles, and James McClay contributed to acquisition, analysis, or interpretation of the data; Jana Ponce, Megan Timmerman, and Corrine Hanson drafted the manuscript; Mariah Jackson, Alfred Jerrod Anzalone, Kristina Bailey, Harlan Sayles, and James McClay critically revised the manuscript; and Jana Ponce, Megan Timmerman, Alfred Jerrod Anzalone, Kristina Bailey, Harlan Sayles, James McClay, and Corrine Hanson agree to be fully accountable for ensuring the integrity and accuracy of the work. All authors read and approved the final manuscript.
CONFLICT OF INTEREST
The author declares no conflict of interest.
Supporting information
Supporting information.
ACKNOWLEDGMENT
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The project described was supported by the National Institute of General Medical Sciences, U54GM104942 and U54GM115458. Other support for this project wasprovided by the NIAAA (R25AA020818 and R24AA019661 to Kristina Bailey) and the Department of Veterans Affairs (I01CX001714 to Corrine Hanson).
Ponce J, Anzalone AJ, Bailey K, et al. Impact of malnutrition on clinical outcomes in patients diagnosed with COVID‐19. J Parenter Enteral Nutr. 2022;1‐11. 10.1002/jpen.2418
This research abstract was selected for recognition and data were presented at the Premier Paper Session and Vars Award Competition (M20) held March 28, 2022, during the ASPEN 2022 Nutrition Science and Practice Conference (Seattle, WA, and Virtual).
REFERENCES
- 1. Mueller AL, McNamara MS, Sinclair DA. Why does COVID‐19 disproportionately affect older people? Aging. 2020;12(10):9959‐9981. 10.18632/aging.103344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Popkin BM, Du S, Green WD, et al. Individuals with obesity and COVID‐19: a global perspective on the epidemiology and biological relationships. Obes Rev. 2020;21(11):e13128. 10.1111/obr.13128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van Zyl‐Smit RN, Richards G, Leone FT. Tobacco smoking and COVID‐19 infection. Lancet Respir Med. 2020;8(7):664‐665. 10.1016/S2213-2600(20)30239-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Knight SR, Ho A, Pius R, et al. Risk stratification of patients admitted to hospital with covid‐19 using the ISARIC WHO Clinical Characterisation Protocol: development and validation of the 4C Mortality Score. BMJ. 2020;370:m3339. 10.1136/bmj.m3339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mete B, Pehlivan E, Gulbas G, Gunen H. Prevalence of malnutrition in COPD and its relationship with the parameters related to disease severity. Int J Chron Obstruct Pulmon Dis. 2018;13:3307‐3312. 10.2147/COPD.S179609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Czapska D, Ostrowska L, Stefanska E, Karczewski J. Assessment of selected vitamins content in daily food rations of obese patients. Article in Polish. Rocz Panstw Zakl Hig. 2009;60(4):381‐383. [PubMed] [Google Scholar]
- 7. Hickson M. Malnutrition and ageing. Postgrad Med J. 2006;82(963):2‐8. 10.1136/pgmj.2005.037564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Choi YJ, Crimmins EM, Kim JK, Ailshire JA. Food and nutrient intake and diet quality among older Americans. Public Health Nutr. 2021;24(7):1638‐1647. 10.1017/S1368980021000586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Dube BP, Laveneziana P. Effects of aging and comorbidities on nutritional status and muscle dysfunction in patients with COPD. J Thorac Dis. 2018;10(suppl 12):S1355‐S1366. 10.21037/jtd.2018.02.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chandra RK. Rosette‐forming T lymphocytes and cell‐mediated immunity in malnutrition. Br Med J. 1974;3(5931):608‐609. 10.1136/bmj.3.5931.608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ferrari‐Baliviera E, Pierdominici S, Sarcinelli L. Effects of the nutritional status on the respiratory system. Article in Italian. Minerva Anestesiol. 1989;55(11):443‐450. [PubMed] [Google Scholar]
- 12. Briguglio M, Pregliasco FE, Lombardi G, Perazzo P, Banfi G. The malnutritional status of the host as a virulence factor for new Coronavirus SARS‐CoV‐2. Front Med (Lausanne). 2020;7:146. 10.3389/fmed.2020.00146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guenter P, Abdelhadi R, Anthony P, et al. Malnutrition diagnoses and associated outcomes in hospitalized patients: United States, 2018. Nutr Clin Pract. 2021;36(5):957‐969. 10.1002/ncp.10771 [DOI] [PubMed] [Google Scholar]
- 14. Ghignone M, Quintin L. Malnutrition and respiratory function. Int Anesthesiol Clin. 1986;24(1):65‐74. 10.1097/00004311-198602410-00007 PubMed PMID: 3081450. [DOI] [PubMed] [Google Scholar]
- 15. Odeyemi Y, Moraes AGD, Gajic O. 15 ‐ What factors predispose patients to acute respiratory distress syndrome? In: Deutschman CS, Neligan PJ. Evidence‐Based Practice of Critical Care. Elsevier; 2020:103‐108.e1. 10.1016/B978-0-323-64068-8.00024-9 [DOI]
- 16. Abate SM, Chekole YA, Estifanos MB, Abate KH, Kabthymer RH. Prevalence and outcomes of malnutrition among hospitalized COVID‐19 patients: a systematic review and meta‐analysis. Clin Nutr ESPEN. 2021;43:174‐183. 10.1016/j.clnesp.2021.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nicolau J, Ayala L, Sanchís P, et al. Influence of nutritional status on clinical outcomes among hospitalized patients with COVID‐19. Clin Nutr ESPEN. 2021;43:223‐229. 10.1016/j.clnesp.2021.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Haendel MA, Chute CG, Bennett TD, et al. The national COVID cohort collaborative (N3C): rationale, design, infrastructure, and deployment. J Am Med Inform Assoc. 2021;28(3):427‐443. 10.1093/jamia/ocaa196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dixon BE, Wen C, French T, Williams JL, Duke JD, Grannis SJ. Extending an open‐source tool to measure data quality: case report on Observational Health Data Science and Informatics (OHDSI). BMJ Health Care Inform. 2020;27(1):e100054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Visweswaran S, Becich MJ, D'itri VS, et al. Accrual to Clinical Trials (ACT): a clinical and translational science award consortium network. JAMIA Open. 2018;1(2):147‐152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Topaloglu U, Palchuk MB. Using a federated network of real‐world data to optimize clinical trials operations. JCO Clin Cancer Inform. 2018;2(2):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bian J, Lyu T, Loiacono A, et al. Assessing the practice of data quality evaluation in a national clinical data research network through a systematic scoping review in the era of real‐world data. J Am Med Inform Assoc. 2020;27(12):1999‐2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Quan H, Li B, Couris CM, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676‐682. [DOI] [PubMed] [Google Scholar]
- 24. Katona P, Katona‐Apte J. The interaction between nutrition and infection. Clin Infect Dis. 2008;46(10):1582‐1588. 10.1086/587658. [DOI] [PubMed] [Google Scholar]
- 25. Mertens E, Penalvo JL. The burden of malnutrition and fatal COVID‐19: a global burden of disease analysis. Front Nutr. 2020;7:619850. 10.3389/fnut.2020.619850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Thomas T, Stefanoni D, Reisz JA, et al. COVID‐19 infection alters kynurenine and fatty acid metabolism, correlating with IL‐6 levels and renal status. JCI Insight . 2020;5(14). 10.1172/jci.insight.140327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Niederer LE, Miller H, Haines KL, et al. Prolonged progressive hypermetabolism during COVID‐19 hospitalization undetected by common predictive energy equations. Clin Nutr ESPEN. 2021;45:341‐350. 10.1016/j.clnesp.2021.07.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Londhe P, Guttridge DC. Inflammation induced loss of skeletal muscle. Bone. 2015;80:131‐142. 10.1016/j.bone.2015.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bedock D, Bel Lassen P, Mathian A, et al. Prevalence and severity of malnutrition in hospitalized COVID‐19 patients. Clin Nutr ESPEN. 2020;40:214‐219. 10.1016/j.clnesp.2020.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shahbazi S, Hajimohammadebrahim‐Ketabforoush M, Vahdat Shariatpanahi M, Shahbazi E, Vahdat, Shariatpanahi Z. The validity of the global leadership initiative on malnutrition criteria for diagnosing malnutrition in critically ill patients with COVID‐19: A prospective cohort study. Clin Nutr ESPEN. 2021;43:377‐382. 10.1016/j.clnesp.2021.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Song F, Ma H, Wang S, et al. Nutritional screening based on objective indices at admission predicts in‐hospital mortality in patients with COVID‐19. Nutr J. 2021;20(1):46. 10.1186/s12937-021-00702-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhao X, Li Y, Ge Y, et al. Evaluation of Nutrition Risk and Its Association With Mortality Risk in Severely and Critically Ill COVID‐19 Patients. JPEN J Parenter Enteral Nutr. 2021;45(1):32‐42. 10.1002/jpen.1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kananen L, Eriksdotter M, Boström AM, et al. Body mass index and Mini Nutritional Assessment‐Short Form as predictors of in‐geriatric hospital mortality in older adults with COVID‐19. Clin Nutr . Accepted manuscript. Published online July 29, 2021. 10.1016/j.clnu.2021.07.025 [DOI] [PMC free article] [PubMed]
- 34. Caccialanza R, Formisano E, Klersy C, et al; NUTRI‐COVID19 Collaborative Working Group . Nutritional parameters associated with prognosis in non‐critically ill hospitalized COVID‐19 patients: the NUTRI‐COVID19 study. Clin Nutr . Accepted manuscript. Published online June 25, 2021. 10.1016/j.clnu.2021.06.020 [DOI] [PMC free article] [PubMed]
- 35. Yanowsky‐Escatell FG, Ontiveros‐Galindo AL, Arellano‐Arteaga KJ, et al. Use of mNUTRIC‐score for nutrition risk assessment and prognosis prediction in critically ill patients with COVID‐19: a retrospective observational study. Crit Care Res Pract. 2021;2021:5866468. 10.1155/2021/5866468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Maruyama T, Fujisawa T, Suga S, et al. Outcomes and prognostic features of patients with influenza requiring hospitalization and receiving early antiviral therapy: a prospective multicenter cohort study. Chest. 2016;149(2):526‐534. 10.1378/chest.14-2768. [DOI] [PubMed] [Google Scholar]
- 37. Banks M, Bauer J, Graves N, Ash S. Malnutrition and pressure ulcer risk in adults in Australian health care facilities. Nutrition. 2010;26(9):896‐901. 10.1016/j.nut.2009.09.024. [DOI] [PubMed] [Google Scholar]
- 38. White JV, Guenter P, Jensen G, et al. Consensus statement: Academy of Nutrition and Dietetics and American Society for Parenteral and Enteral Nutrition: characteristics recommended for the identification and documentation of adult malnutrition (undernutrition). JPEN J Parenter Enteral Nutr. 2012;36(3):275‐283. 10.1177/0148607112440285. [DOI] [PubMed] [Google Scholar]
- 39. Cruz‐Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39(4):412‐423. 10.1093/ageing/afq034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schleithoff SS, Zittermann A, Tenderich G, Berthold HK, Stehle P, Koerfer R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: a double‐blind, randomized, placebo‐controlled trial. Am J Clin Nutr. 2006;83(4):754‐759. 10.1093/ajcn/83.4.754. [DOI] [PubMed] [Google Scholar]
- 41. Huguet N, Schmidt T, Larson A, et al. Prevalence of pre‐existing conditions among community health center patients with COVID‐19: implications for the Patient Protection and Affordable Care Act. J Am Board Fam Med. 2021;34(suppl):S247‐S249. 10.3122/jabfm.2021.S1.200571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tobert CM, Mott SL, Nepple KG. Malnutrition diagnosis during adult inpatient hospitalizations: analysis of a multi‐institutional collaborative database of academic medical centers. J Acad Nutr Diet. 2018;118(1):125‐131. 10.1016/j.jand.2016.12.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting information.


