Figure 3.
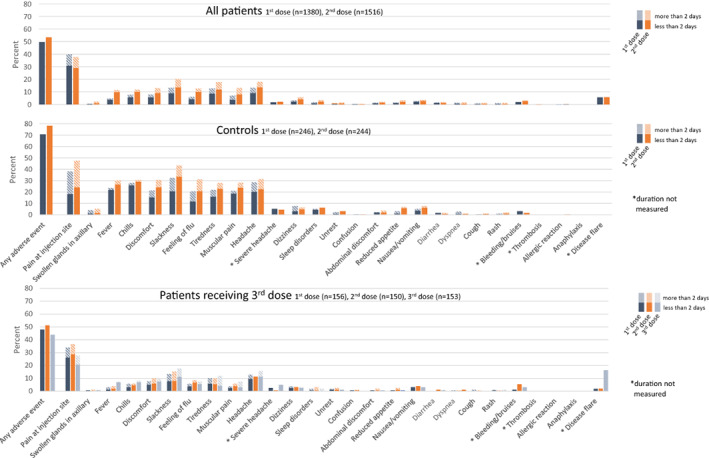
Type and duration of adverse events reported after doses 1 (blue bars) and 2 (orange bars) of SARS–CoV‐2 vaccine among patients with immune‐mediated inflammatory disease (IMID) and healthy controls and after dose 3 (gray bars) among IMID patients who had a weak serologic response (defined as <70 arbitrary units per milliliter) to doses 1 and 2. Adverse events were reported for all patients and a subset of 246 healthy controls described in Patients and Methods. Color figure can be viewed in the online issue, which is available at http://onlinelibrary.wiley.com/doi/10.1002/art.42153/abstract.
