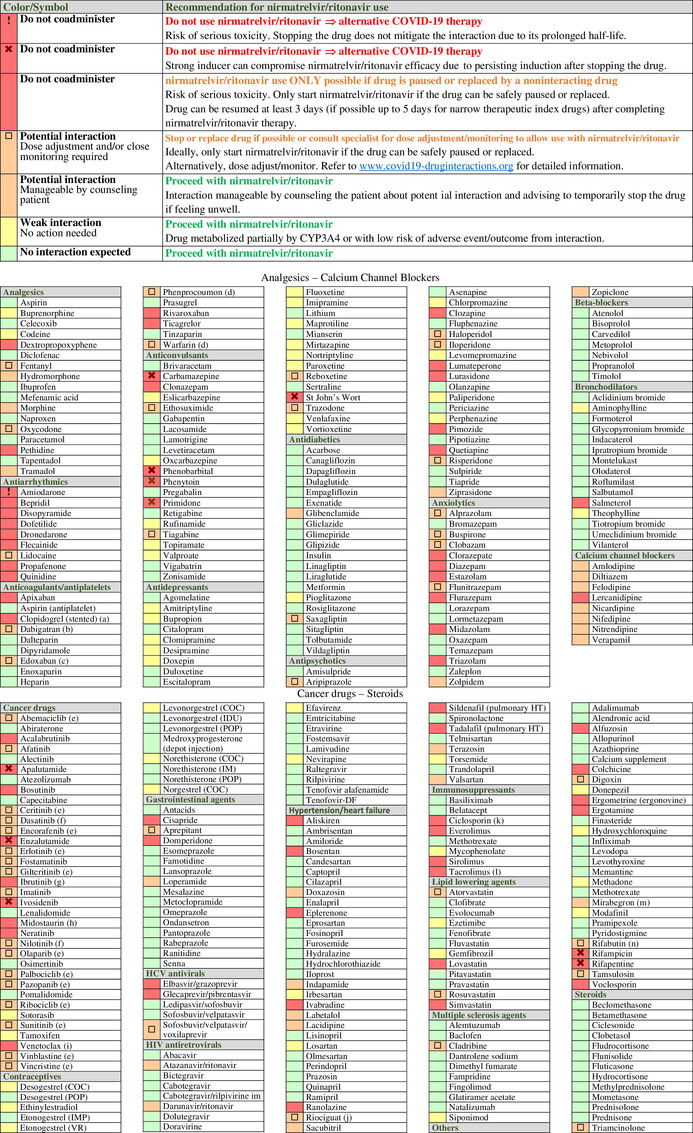
Table 1.
Potential drug–drug interactions (DDIs) between nirmatrelvir/ritonavir and comedications classified by therapeutic classes. DDIs are categorized as: red flag for drugs that should not be coadministered due to the risk of deleterious adverse outcomes; amber flag for potential clinical DDIs manageable by monitoring or dosage adjustment; yellow flag for DDIs of weak clinical relevance with no need for monitoring or dosage adjustment; and green flag for no DDI. Symbols are used to distinguish red flag DDIs which cannot be prevented by stopping a comedication. This is notably the case for drugs characterized by a very long elimination half‐life or for strong inducers due to their prolonged induction upon discontinuation of the drug. Use of such medications would require an alternative COVID‐19 treatment. A symbol is also used for amber DDIs that may require a complex/impractical monitoring that may not be feasible outside a clinical setting. If such comedications cannot be stopped or replaced, the risk–benefit of prescribing nirmatrelvir/ritonavir should be evaluated. Amber DDIs without a symbol are manageable by counseling patients about potential DDIs and advising them to temporarily pause the comedication if feeling unwell, thus enabling treatment with nirmatrelvir/ritonavir. The list of drugs is incomplete, detailed information and additional DDIs are available at https://www.covid19‐druginteractions.org. 23
|
(a) Ritonavir reduces the conversion to clopidogrel's active metabolite, leading to insufficient inhibition of platelet aggregation. 35 Thus, it is recommended to avoid nirmatrelvir/ritonavir in patients at very high risk of thrombosis (e.g., early period post coronary stenting), 37 unless clopidogrel can be switched to the noninteracting drug prasugrel. However, nirmatrelvir/ritonavir treatment is possible in other clinical situations for which a transient loss in clopidogrel efficacy is acceptable (e.g., alternative to aspirin in intolerant patients).
(b) Reduce to 110 mg twice daily in individuals with normal renal function and to 75 mg twice daily in individuals with moderate renal impairment.
(c) Reduce edoxaban to 30 mg.
(d) Monitor international normalized ratio as clinically indicated.
(e) Decision to hold or dose adjust the cancer drug should be made in conjunction with the patient's oncologist. Consult www.covid19‐druginteractions.org 23 for details related to dosage adjustment.
(f) Accelerated or blast phase chronic myelogenous leukemia: do not coadminister; use alternative COVID‐19 therapy. In the indication of chronic phase chronic myelogenous leukemia, the decision to hold or dose adjust the cancer drug should be made in conjunction with the patient's oncologist. If it is decided to hold treatment, restart the cancer drug at least 3 days after completing nirmatrelvir/ritonavir. Alternatively dose adjust; consult www.covid19‐druginteractions.org 23 for details.
(g) The decision to hold ibrutinib treatment should be made in conjunction with the patient's oncologist. It may be dangerous to interrupt therapy in patients with high‐volume chronic lymphocytic leukemia or mantle cell lymphoma due to disease flare and/or cytokine release. Consider an alternative COVID‐19 therapy.
(h) Strong CYP3A4 inhibitors can substantially increase midostaurin exposure. Consider an alternative COVID‐19 treatment.
(i) Coadministration with nirmatrelvir/ritonavir is contraindicated at initiation and during the dose‐titration phase to minimize the risk of tumor lysis syndrome. Use an alternative COVID19 therapy.
(j) The European product label for riociguat does not recommend its use in the presence of strong inhibitors; the US product label recommends to start riociguat at a dose of 0.5 mg three times daily and to monitor for signs and symptoms of hypotension.
(k) The management of this interaction is challenging and would require dosage adjustment and therapeutic drug monitoring (TDM) of ciclosporin, which may not be possible given the short duration of nirmatrelvir/ritonavir treatment. An alternative COVID treatment should be considered. However, if TDM is available, an empiric dose reduction of ciclosporin has been suggested (reduce total daily dose by 80% and administer once daily) during treatment with nirmatrelvir/ritonavir (Days 1–5). Ciclosporin concentrations should be assessed on Day 6 or 7 and repeated every 2–4 days. 20
(l) The management of this interaction is challenging and would require a substantial reduction in tacrolimus dosage. Considering the complex management of this interaction, an alternative COVID treatment will need to be considered. However, if TDM for tacrolimus is available, it has been suggested to withhold all tacrolimus doses during treatment with nirmatrelvir/ritonavir (Days 1–5). It is advised to measure tacrolimus concentrations on Day 3 to assess the need for a one‐time tacrolimus dose during nirmatrelvir/ritonavir treatment. Tacrolimus concentrations should be assessed on Day 6 or 7 (and every 2–4 days thereafter) and concentrations used to guide the continued withholding or gradual reintroduction of tacrolimus. 20
(m) No dose reduction or monitoring in patients with normal renal function.
(n) Rifabutin is dosed at 150 mg once daily with nirmatrelvir/ritonavir.
COC, combined oral contraceptive; CYP, cytochrome P450; IM, intramuscular depot injection; IMP, implant; IUD, intrauterine device; POP, progestogen‐only pill; VR, vaginal ring.
