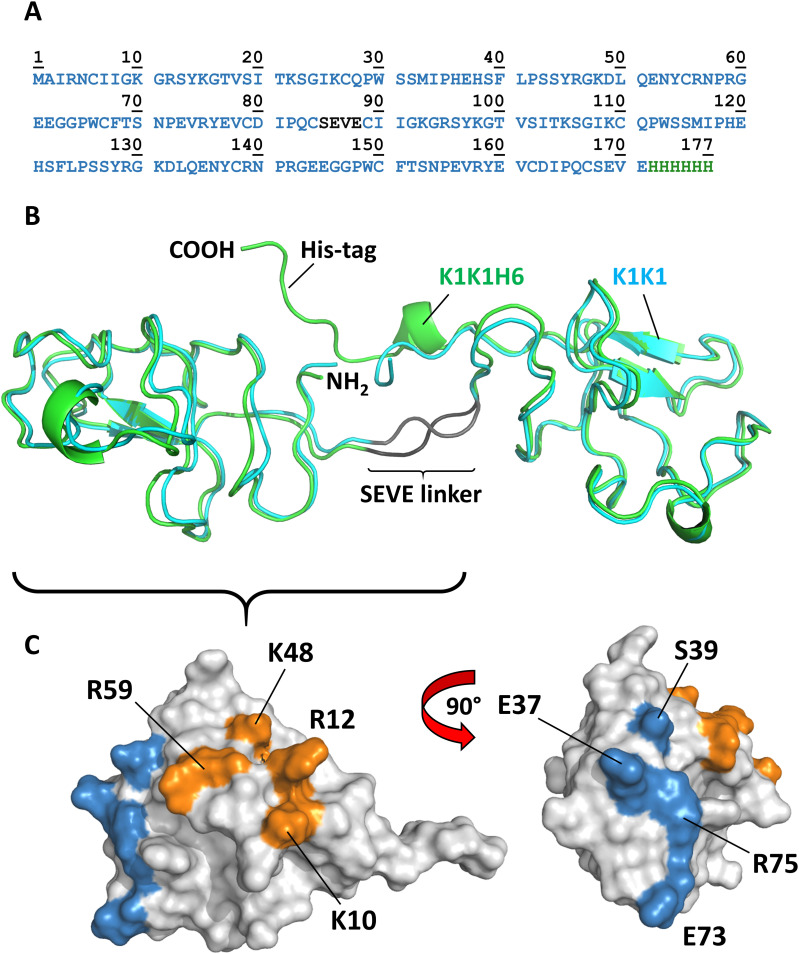
Figure 3. Amino acid sequence, overall structure, and location of binding sites.
(A) Amino acid sequence of K1K1H6 with SEVE linker in dark grey and poly-histidine tag in green. (B) The crystal structure of the two molecules of K1K1 (cyan) and K1K1H6 (green) showing the straight conformation and the N-terminus (NH2) and C-terminus (COOH) located centrally in the linker region and a nearly identical overall structure with a root mean square deviation ranging from 0.8 to 1.8 Å. The C-terminal poly-histidine tag of K1K1H6 is making contacts with residues in the N-terminal kringle domain. (C) Surface representations of single kringle domains of K1K1 showing the location of the residues involved in MET binding, as defined by Lokker et al (1994) 40, shown in blue. The more lateral position of the heparin-binding site is shown in orange with the residues targeted by reverse-charge mutation in K1K1S2 (K10E, R12E) and K1K1S4 (K10E, R12E, K48E, R59E) indicated.