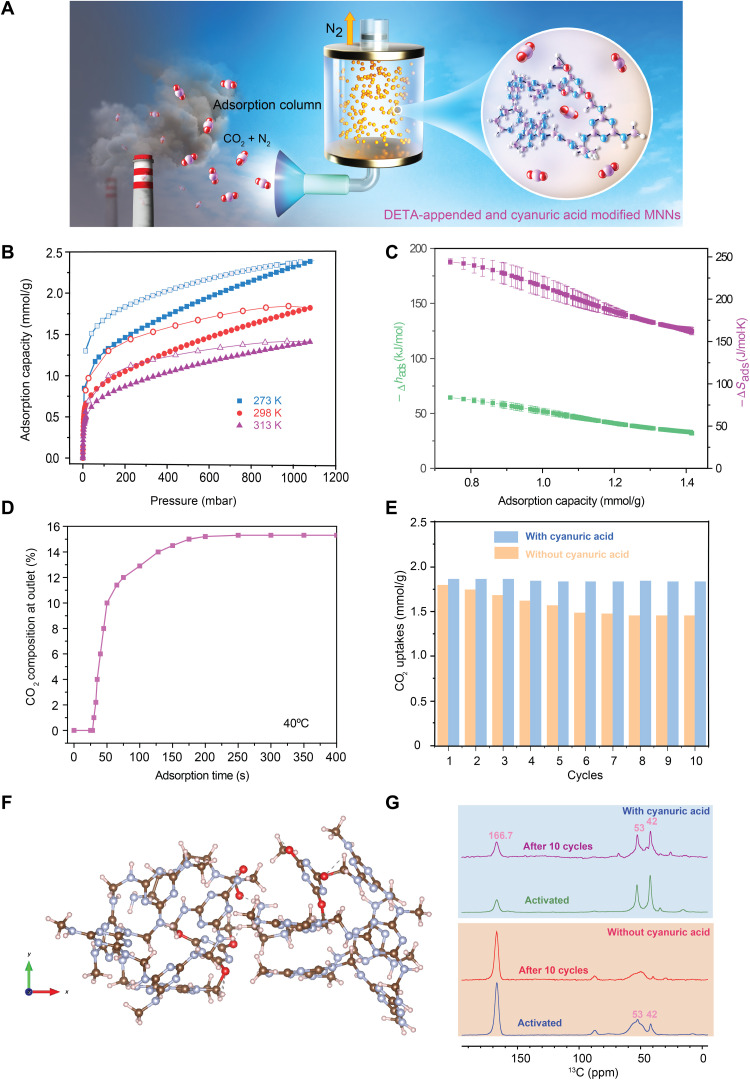
Fig. 5. DFT-calculated structures, kinetics of adsorption, and stability of MNNsCya ⊃ DETA during CO2 cycles.
(A) Schematic diagram of adsorption column system. (B) Adsorption (filled shapes) and desorption (open shapes) isotherms for CO2 uptake in MNNsCya ⊃ DETA at 273, 298, and 313 K. (C) The −Δhads (enthalpy) and −Δsads (entropy) for MNNsCya ⊃ DETA determined using the Clausius-Clapeyron equation as a function of CO2 loading. (D) Adsorption breakthrough and rate for adsorption column of MNNsCya ⊃ DETA at 313 K (15% CO2, 83% N2, and 2% H2O). (E) Recorded CO2 uptakes during 10 adsorption-desorption cycles using the homemade dosing setup. (F) Proposed mixed chemisorption structure with ammonium carbamate pair. The structure was obtained by DFT modeling at the TPSS-D3(BJ)/6-31G* level. (G) 13C NMR (16.4 T) spectra acquired by CP for MNNs ⊃ DETA and MNNsCya ⊃ DETA, before and after 10 CO2 adsorption-desorption cycles.