Abstract
Background
Epidemiological studies are necessary to explore the effect of current pneumococcal conjugate vaccines (PCVs) against antibiotic resistance, including the rise of non-vaccine serotypes that are resistant to antibiotics. Hence, epidemiological changes in the antimicrobial pattern of Streptococcus pneumoniae before and during the first year of the COVID-19 pandemic were studied.
Methods
In this national surveillance study, we characterised the antimicrobial susceptibility to a panel of antibiotics in 3017 pneumococcal clinical isolates with reduced susceptibility to penicillin during 2004–20 in Spain. This study covered the early and late PCV7 periods; the early, middle, and late PCV13 periods; and the first year of the COVID-19 pandemic, to evaluate the contribution of PCVs and the pandemic to the emergence of non-vaccine serotypes associated with antibiotic resistance.
Findings
Serotypes included in PCV7 and PCV13 showed a decline after the introduction of PCVs in Spain. However, an increase in non-PCV13 serotypes (mainly 11A, 24F, and 23B) that were not susceptible to penicillin promptly appeared. A rise in the proportion of pneumococcal strains with reduced susceptibility to β-lactams and erythromycin was observed in 2020, coinciding with the emergence of SARS-CoV-2. Cefditoren was the β-lactam with the lowest minimum inhibitory concentration (MIC)50 or MIC90 values, and had the highest proportion of susceptible strains throughout 2004–20.
Interpretation
The increase in non-PCV13 serotypes associated with antibiotic resistance is concerning, especially the increase of penicillin resistance linked to serotypes 11A and 24F. The future use of PCVs with an increasingly broad spectrum (such as PCV20, which includes serotype 11A) could reduce the impact of antibiotic resistance for non-PCV13 serotypes. The use of antibiotics to prevent co-infections in patients with COVID-19 might have affected the increased proportion of pneumococcal-resistant strains. Cefotaxime as a parenteral option, and cefditoren as an oral choice, were the antibiotics with the highest activity against non-PCV20 serotypes.
Funding
The Spanish Ministry of Science and Innovation and Meiji-Pharma Spain.
Translation
For the Spanish translation of the abstract see Supplementary Materials section.
Introduction
Invasive pneumococcal disease and community-acquired bacterial pneumonia are infectious diseases of high priority for prevention as they are associated with high morbidity and mortality rates.1, 2 Streptococcus pneumoniae (also known as pneumococcus) is the most common cause of community-acquired bacterial pneumonia and is one of the most frequent causes of bacterial meningitis and sepsis.1, 2 Pneumococcal conjugate vaccine (PCV) use is the best prophylactic strategy to prevent invasive pneumococcal disease and community-acquired bacterial pneumonia in children,2 although several clinical trials have also shown great effectiveness in the adult population.3, 4 In Spain, PCV7 was first used in 2001, although mainly in private practice, and vaccine coverage was less than 50% before 2006.5 PCV10 was authorised for use in 2009, but promptly replaced by PCV13 in 2010. PCV13 was highly prescribed by paediatricians and in 2016 was included in the national immunisation schedule of the Spanish public health system, leading to high vaccine coverage rates. In adults, pneumococcal vaccine coverage rates are not made public, although in 2018 they were 22% for Spanish regions that used PCV13, and 26% for those that used pneumococcal polysaccharide vaccine (PPV)23.5 A marked reduction in the incidence of invasive pneumococcal disease caused by PCV13 serotypes has been reported in Spain, not only in children but also in adults, owing to the herd-immunity effects of paediatric vaccination.5 Another important benefit of using PCVs is their contribution to lowering the burden of antimicrobial resistance, by controlling serotypes that have reduced susceptibility.6 However, an increase in non-PCV13 serotypes, mainly in adults, might jeopardise the effectiveness of this vaccine.5, 7
Research in context.
Evidence before this study
We searched PubMed on Jan 2, 2021 for studies in children and adults published between Jan 1, 2004, and Dec 31, 2020, using the terms “invasive pneumococcal disease” and/or “serotypes”, and “pneumococcal conjugate vaccines”, and “antibiotic resistance”, and “SARS-CoV-2”, with no language restrictions. We screened the search results, which included population-based studies and observational studies related to the epidemiology of invasive pneumococcal disease caused by antimicrobial-resistant strains affecting adults before and after the introduction of pneumococcal conjugate vaccines (PCVs). Overall, studies that included countries that had introduced PCVs in childhood immunisation programmes reported a reduction in overall invasive pneumococcal disease, and a decline in incidence by vaccine serotypes, including serotypes with antibiotic resistance. Herd-immunity protection in adults has been observed in countries with long-term use of PCVs in children, upholding the importance of indirect protection conferred by PCVs. The replacement of serotypes after PCV13 has been subject to geographical discrepancies globally.
Added value of this study
Different PCVs have been used since 2001 (with the introduction of PCV7, followed by PCV13) and it is important to determine the effect of these vaccines on the epidemiology of resistant strains. Such knowledge is especially important given the COVID-19 pandemic, in which many antibiotics have been prescribed at hospital and community level to prevent the potential risk of co-infection by bacterial pathogens, and the resulting threat of increased resistance is of concern. In this national-level longitudinal study in Spain, 2004–20, we evaluated the evolution of pneumococcal strains resistant to a range of antibiotics, including penicillin, amoxicillin, cefotaxime, erythromycin, levofloxacin, and third-generation oral cephalosporins such as cefixime, cefpodoxime, and cefotaxime. We also analysed the patterns of antibiotic resistance before and during the first year of the COVID-19 pandemic, to see if there were variations that might be attributable to the use of antibiotics to prevent co-infections in patients infected by SARS-CoV-2.
Implications of all the available evidence
The study shows a reduction in vaccine-serotypes displaying antibiotic resistance after the introduction of PCV7 and PCV13, confirming the importance of these vaccines in controlling the problem of antibiotic resistance. However, a rise in non-PCV13 serotypes that harbour resistance (including 11A, 24F, and 23B), has been observed in the past 5 years. Future vaccines that contain additional serotypes associated with antibiotic resistance will partly solve this problem by increasing potential coverage against some of these emerging non-vaccine serotypes. Our data suggest that the increased proportion of resistant strains during the first year of the COVID-19 pandemic should be taken into consideration regarding the use of antibiotics as a routine strategy to prevent bacterial co-infections, as such use could exacerbate the problem of antibiotic resistance.
There have been constant increases in serotypes associated with antimicrobial resistance, with declines in susceptibility rates after the introduction of PCVs and increases in non-PCV serotypes after PCVs were implemented in the paediatric population.8 In addition, the emergence of multidrug-resistant serotype 19A isolates was reported shortly after the introduction of PCV7 globally.8 This occurrence is consistent with a 2011 report9 that explored antimicrobial resistance rates in S pneumoniae globally, which showed that susceptibility rates had decreased throughout the years in particular regions. Therefore, we did a national longitudinal study to characterise the evolution of antibiotic susceptibility throughout 16 years (2004–20), with a special focus on third-generation oral cephalosporins, because in Spain these antibiotics are widely used to treat patients with pneumonia who have not been hospitalised. Another major goal of our study was to evaluate the contributions of PCV7, PCV13, and the COVID-19 pandemic to the emergence of non-vaccine serotypes that are associated with antibiotic resistance. Studies published in the past 2 years suggested that S pneumoniae could interact with SARS-CoV-2.10, 11 Vaccination with PCV13 has been associated with a reduced risk of COVID-19 diagnosis, hospitalisation, and mortality in patients infected by SARS-CoV-2.10 Furthermore, pneumococcal carriage has been linked with impaired anti-SARS-CoV-2 immune responses, affecting mucosal IgA concentrations in individuals with mild or asymptomatic infection and the cellular memory responses in most patients who are infected.11 Hence, vaccination using PCVs that reduces both the duration of and the number of people in the carrier state could preserve the immune response against SARS-CoV-2 and be the reason for a lowered risk of COVID-19.10, 11
Methods
Study design
In this national surveillance study, we characterised 3017 clinical isolates that were non-susceptible to penicillin, which we received at the Spanish Pneumococcal Reference Laboratory (Madrid, Spain) in 2004–20. These isolates were from adult patients hospitalised with invasive pneumococcal disease or non-bacteraemic pneumococcal pneumonia. We did not include strains from adults with meningitis. We also analysed the effect of PCVs in the epidemiology of S pneumoniae strains with reduced susceptibility to penicillin assessed at different time periods. We compared 2019 (pre-COVID-19) and 2020 (COVID-19) to analyse the effect of SARS-CoV-2 in the antimicrobial susceptibility of S pneumoniae (appendix 2, pp 2–3).
We included around 500 clinical isolates that were non-susceptible to penicillin and had a minimum inhibitory concentration (MIC) of at least 0·12 μg/mL from 2004 (early PCV7 period), 2008 (late PCV7 period), 2012 (early PCV13 period), 2016 (middle PCV13 period), 2019 (late PCV13 and pre-COVID-19 period), and 2020 (COVID-19 period; appendix 2, p 3). These strains were obtained using our collection programme at the Spanish Pneumococcal Reference Laboratory for strains from hospitals distributed throughout the entire country. To avoid possible bias, we did a random selection using the RAND function in Microsoft Excel (2016 [Windows]) from our database of collected strains to ensure a general distribution from around the country.
This study was done as a public health investigation with internal approval from Instituto de Salud Carlos III (Madrid, Spain) for the characterisation of pneumococcal strains; as such, external ethics committee approval was not required.
Characterisation of pneumococcal serotypes and antibiotic susceptibility
Serotyping was done by Quellung reaction and dot blot assay using specific antisera, or PCR sequencing.5, 12 For antimicrobial susceptibility, we analysed different β-lactam antibiotics that included penicillin, amoxicillin, cefotaxime, cefditoren, cefixime, and cefpodoxime. We also analysed other antibiotic groups, such as erythromycin as a representative macrolide and levofloxacin to represent fluoroquinolones. PCV7 vaccine contains serotypes 4, 6B, 9V, 14, 18C, 19F, and 23F. PCV13 vaccine contains PCV7 serotypes plus 1, 3, 5, 6A, 7F, and 19A. PCV15 vaccine contains PCV13 serotypes plus 22F and 33F, and PCV20 contains PCV15 serotypes plus 8, 10A, 11A, 12F, and 15B.
Antibiotic susceptibility was evaluated by the test diffusion method and the MIC values were determined by the agar dilution technique in accordance with the European Committee on Antimicrobial Susceptibility Testing (EUCAST) criteria, using EUCAST breakpoint recommendations for data interpretation.8 For those antibiotics without a defined breakpoint by EUCAST or the Clinical and Laboratory Standards Institute such as cefixime and cefditoren, we used the same breakpoints as cefotaxime (appendix 2, p 4).
Statistical analysis
Statistical analysis was done by using a two-tailed Student's t-test (for two-group comparisons), and ANOVA followed by Dunnett's post-hoc test was used for multiple comparisons. The effect of vaccination against resistant serotypes was calculated by comparing the incidence rates of resistant strains during the different periods and calculating the incidence rate ratio (IRR) with 95% CI using Poisson regression models. The effect of SARS-CoV-2 in the rise of pneumococcal-resistant strains was measured using Fisher's exact test. GraphPad InStat (version 8.0; GraphPad Software, San Diego, CA, USA) was used for statistical analysis. Differences were considered significant if p<0·05 and highly significant if p<0·01 or p<0·001.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
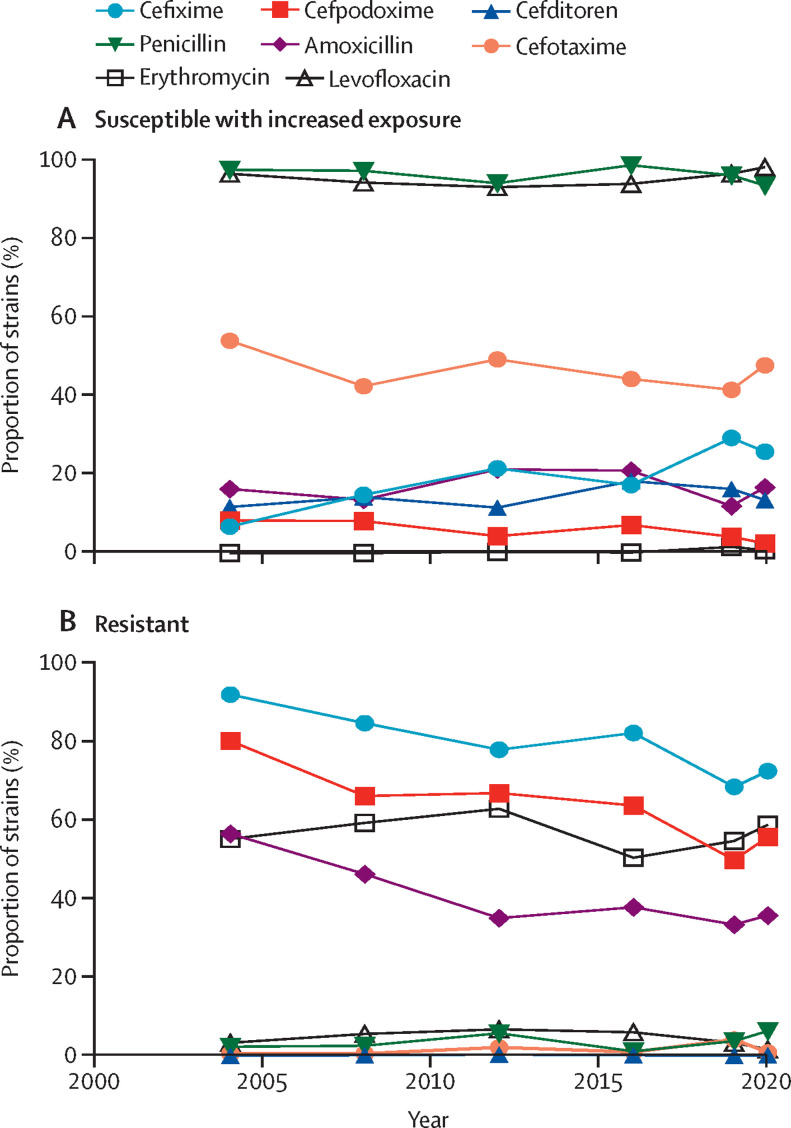
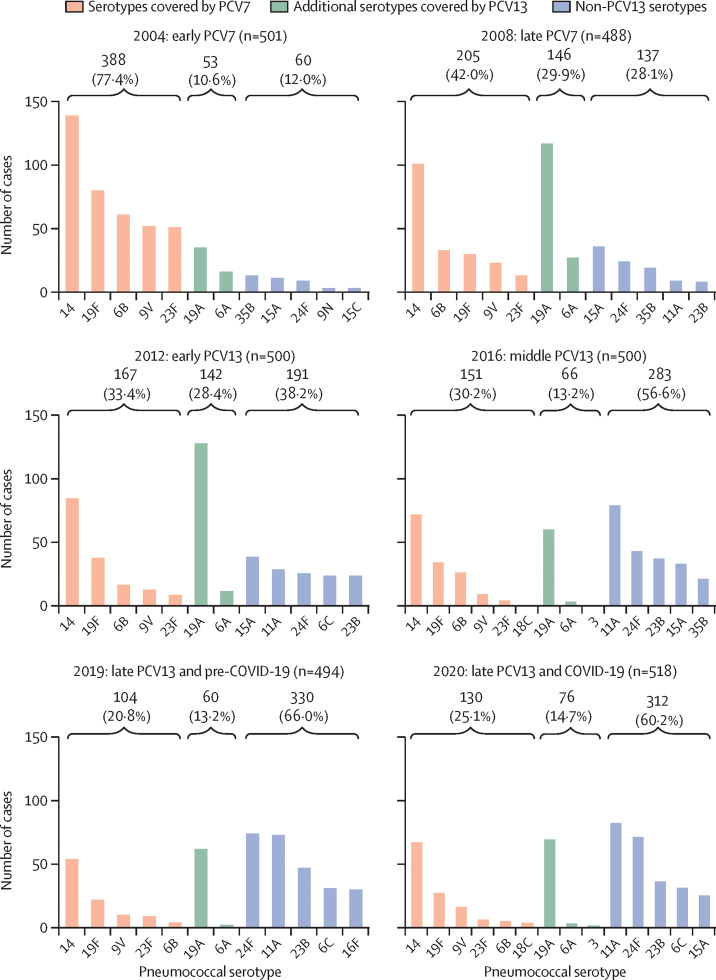
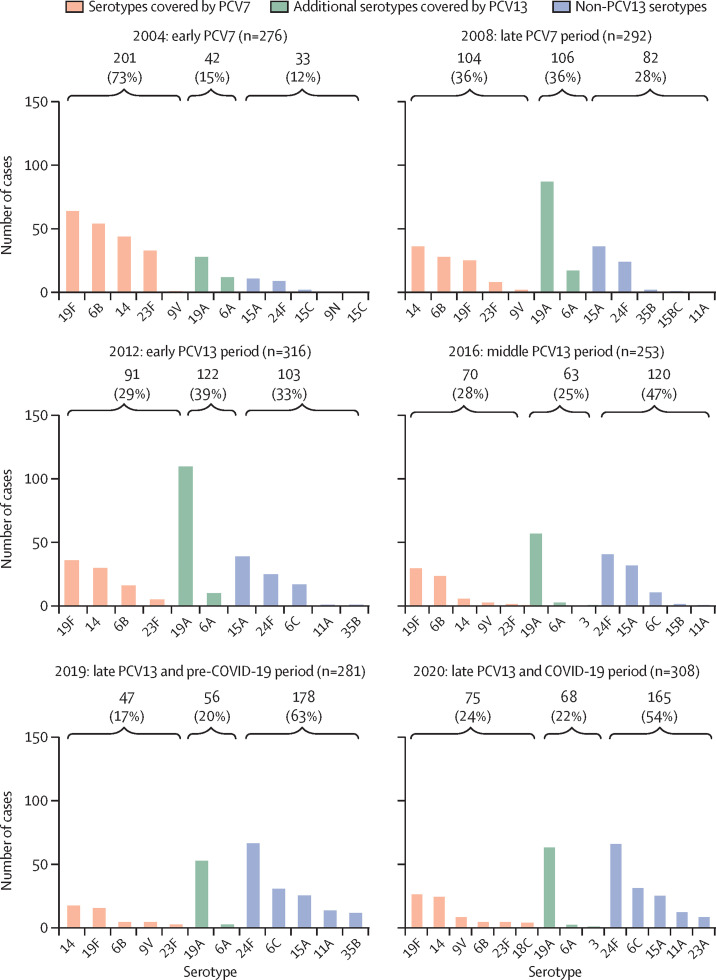
To characterise the evolution of the clinical isolates by their susceptibility pattern 2004–20, we used EUCAST criteria to categorise the strains as fully susceptible, susceptible with increased exposure, or resistant (figure 1 ; appendix 2, p 4). With cefotaxime, more than 40% of strains were susceptible with increased exposure, followed by (in order of declining percentages) cefixime, amoxicillin, cefditoren, cefpodoxime and erythromycin (figure 1A). Among resistant strains, the antibiotic associated with the highest proportion of resistant strains was cefixime (>68%), followed by cefpodoxime (>50%), erythromycin (>50%), and amoxicillin (>33%; figure 1B). In addition, the antibiotics showing the lowest proportion of resistant strains during the study period were cefditoren (<0·4%), cefotaxime (<5%), penicillin (<6·5%), and levofloxacin (<7%; figure 1B). A decrease in the proportion of resistant strains was observed after the introduction of PCVs (ie, in the late PCV7 and early-to-middle PCV13 periods), suggesting that these vaccines were effective in controlling the emergence of resistant strains. However, a trend towards a moderate increase in the proportion of resistant strains was observed for some antibiotics in the late PCV13 period (figure 1B). A comparison of the 2019 (pre-COVID-19) and 2020 (COVID-19) periods showed an increase (p<0·05) in the proportion of strains that were resistant to various antibiotics, such as penicillin (3% in 2019 vs 6% in 2020), amoxicillin (33% vs 36%), cefixime (68% vs 72%), cefpodoxime (50% vs 56%), and erythromycin (55% vs 59%), but showed no differences for cefditoren or levofloxacin (figure 1B). For cefotaxime, which is widely used in Spanish hospitals as a parenteral antibiotic against respiratory and systemic infection, we also found an increase in the proportion of strains with reduced susceptibility during the first COVID-19 pandemic year (42% in 2019 vs 48% in 2020; figure 1A). Cefditoren was the antibiotic showing the highest proportion of susceptible strains (>81%), followed by cefotaxime (>45%) and erythromycin (>37%; appendix 2, p 5). By contrast, cefixime, followed by cefpodoxime, had the lowest proportion of susceptible strains (appendix 2, p 5). We evaluated the contribution of pneumococcal vaccination (PCV7 until 2009 and PCV13 since 2010) to the national epidemiology of pneumococcal strains with reduced susceptibility to penicillin and either reduced susceptibility with increased exposure or resistance to erythromycin (Figure 2, Figure 3 ). The susceptibility with increased exposure or resistance of pneumococcal serotypes included in PCV7 and PCV13 decreased in the middle and late periods after the introduction of these vaccines (PCV7: IRR 0·31 [95% CI 0·26–0·38] for penicillin vs 0·35 [0·27–0·46] for erythromycin; PCV13: 0·37 [0·32–0·43] for penicillin vs 0·38 [0·31–0·47] for erythromycin). Serotype 14 accounted for the highest proportion of non-susceptible strains, showing a constant and steady trend in the last 5 years of the study period, especially for penicillin (Figure 2, Figure 3). A reduction between 2004 and 2020 in PCV13 strains with susceptibility with increased exposure or resistance to penicillin (88% in 2004 vs 40% in 2020) and erythromycin (88% vs 46%) was obtained after the introduction of these PCVs, strengthening the importance of these vaccines in the fight against antibiotic resistance (Figure 2, Figure 3). In the case of erythromycin, because we selected strains that had penicillin susceptibility with increased exposure or resistance, a limitation of our study was that we did not measure the effect of PCVs against strains that were fully susceptible to penicillin, but resistant to erythromycin. Also, an increase in non-susceptible strains belonging to serotype 19A was observed from 2008, coinciding with the late-PCV7 period (Figure 2, Figure 3). Hence, the use of PCV13 enabled the control of serotype 19A strains that had reduced susceptibility to penicillin and erythromycin, although in the past 5 years, a situation of stability was observed for both penicillin and erythromycin (Figure 2, Figure 3). We found an increase in non-PCV13 strains that had susceptibility with increased exposure or resistance since the introduction of both of these PCVs (12% in 2004 vs 54% in 2020 for erythromycin and 12% vs 60% for penicillin; Figure 2, Figure 3). With non-PCV13 serotypes, we observed an increase of serotype 11A strains that were not susceptible to penicillin and an increase of serotype 24F strains that were not susceptible to penicillin or erythromycin (Figure 2, Figure 3). With penicillin resistance, currently serotype 11A, followed by serotype 24F, are the two most frequent causes of pneumococcal disease caused by non-susceptible strains, and account for 30% of all cases associated with reduced susceptibility to penicillin. For simultaneous resistance to penicillin and erythromycin, epidemiological data suggested that serotype 24F was responsible for 24% of all cases, with a secondary role for serotype 11A, as only 5% of cases caused by this serotype had resistance to both antibiotics.
Figure 1.
Evolution of Streptococcus pneumoniae strains based on their susceptibility to antibiotics, 2004–20
Strains were categorised using European Committee on Antimicrobial Susceptibility Testing criteria as susceptible with increased exposure to antibiotics (A) or resistant to antibiotics (B).
Figure 2.
Evolution of pneumococcal serotypes with reduced susceptibility to penicillin, 2004–20
The number and proportion of pneumococcal cases caused by serotypes covered by PCV7, additional serotypes covered by PCV13, or not included in PCV13 (the top five or five most frequent) that were either susceptible with increased exposure or resistant to penicillin. Reduced susceptibility was defined as a minimum inhibitory concentration of 0·12 μg/mL or higher. PCV=pneumococcal conjugate vaccine.
Figure 3.
Evolution of pneumococcal serotypes with reduced susceptibility to erythromycin among strains that are either susceptible with increased exposure or resistant to penicillin, 2004–20
The number and proportion of pneumococcal cases caused by serotypes covered by PCV7, additional serotypes covered by PCV13, or not included in PCV13 (the top five or five most frequent) that were either susceptible with increased exposure or resistant to penicillin or erythromycin. Reduced susceptibility was defined as a minimum inhibitory concentration of 0·5 μg/mL or higher. PCV=pneumococcal conjugate vaccine.
To evaluate the effect of PCVs and SARS-CoV-2 in the MIC values to β-lactams, we explored the evolution of MIC50 and MIC90, analysing the three most prevalent PCV13 serotypes (19A, 14, and 19F) and non-PCV13 serotypes (11A, 24F, and 23B) associated with reduced susceptibility (ie, susceptibility with increased exposure or resistance; table ). Among third-generation oral cephalosporins, cefixime had the highest MIC50 and MIC90 values, irrespective of the serotype, followed by cefpodoxime, whereas cefditoren was the most active cephalosporin showing the lowest MIC50 or MIC90 value, which was even lower than for cefotaxime—one of the most widely used parenteral cephalosporins against invasive pneumococcal disease (table). Overall, these MIC50 or MIC90 values indicated that cefotaxime, and cefditoren to a greater extent, were the β-lactam antibiotics with the highest activity against the most frequent serotypes with susceptibility with increased exposure or resistance to penicillin. Hence, our results showed that cefditoren achieved the lowest MIC values between 2004 and 2020, which was significant compared with each β-lactam antibiotic, including cefotaxime (p<0·001, two-tailed Student t-test) and even if multiple comparisons were done with oral cephalosporins such as cefixime and cefpodoxime (p<0·01 for one-way ANOVA followed by Dunnett's post-hoc test). In addition, PCV13 serotypes (19A, 14, and 19F), and serotype 11A as a non-PCV13 serotype, had higher MIC50 or MIC90 values than all of these cephalosporins compared with serotypes 24F and 23B.
Table.
MIC50and MIC90values for the three most prevalent PCV13 and non-PCV13 serotypes against different β-lactam antibiotics, 2004–20
|
2004 |
2008 |
2012 |
2016 |
2019 |
2020 |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MIC50 | MIC90 | MIC50 | MIC90 | MIC50 | MIC90 | MIC50 | MIC90 | MIC50 | MIC90 | MIC50 | MIC90 | |
| Serotype 19A, mg/L | ||||||||||||
| Cefixime | 16·00 | 16·00 | 16·00 | 16·00 | 16·00 | 16·00 | 16·00 | 16·00 | 16·00 | 16·00 | 16·00 | 16·00 |
| Cefpodoxime | 2·00 | 2·00 | 2·00 | 4·00 | 2·00 | 4·00 | 4·00 | 4·00 | 4·00 | 4·00 | 2·00 | 4·00 |
| Cefditoren | 0·50 | 0·50 | 0·50 | 1·00 | 0·50 | 1·00 | 1·00 | 1·00 | 1·00 | 1·00 | 0·50 | 1·00 |
| Penicillin | 0·50 | 1·00 | 1·00 | 2·00 | 2·00 | 4·00 | 1·00 | 2·00 | 2·00 | 2·00 | 2·00 | 2·00 |
| Amoxicillin | 0·50 | 2·00 | 0·50 | 4·00 | 2·00 | 4·00 | 2·00 | 4·00 | 4·00 | 4·00 | 2·00 | 4·00 |
| Cefotaxime | 0·50 | 1·00 | 0·50 | 1·00 | 1·00 | 2·00 | 1·00 | 2·00 | 2·00 | 4·00 | 2·00 | 2·00 |
| Serotype 14, mg/L | ||||||||||||
| Cefixime | 16·00 | 16·00 | 16·00 | 16·00 | 16·00 | 16·00 | 16·00 | 16·00 | 16·00 | 16·00 | 16·00 | 16·00 |
| Cefpodoxime | 2·00 | 4·00 | 2·00 | 4·00 | 2·00 | 4·00 | 2·00 | 4·00 | 2·00 | 4·00 | 2·00 | 4·00 |
| Cefditoren | 0·50 | 1·00 | 0·50 | 1·00 | 0·50 | 1·00 | 1·00 | 1·00 | 1·00 | 2·00 | 0·50 | 1·00 |
| Penicillin | 1·00 | 2·00 | 1·00 | 2·00 | 1·00 | 2·00 | 1·00 | 2·00 | 2·00 | 4·00 | 2·00 | 2·00 |
| Amoxicillin | 2·00 | 8·00 | 2·00 | 8·00 | 1·00 | 4·00 | 1·00 | 4·00 | 1·00 | 8·00 | 1·00 | 2·00 |
| Cefotaxime | 1·00 | 2·00 | 1·00 | 2·00 | 1·00 | 2·00 | 1·00 | 2·00 | 2·00 | 4·00 | 1·00 | 2·00 |
| Serotype 19F, mg/L | ||||||||||||
| Cefixime | 8·00 | 16·00 | 8·00 | 16·00 | 16·00 | 16·00 | 16·00 | 16·00 | 8·00 | 16·00 | 16·00 | 16·00 |
| Cefpodoxime | 1·00 | 2·00 | 1·00 | 2·00 | 2·00 | 4·00 | 2·00 | 4·00 | 1·00 | 2·00 | 2·00 | 4·00 |
| Cefditoren | 0·25 | 0·50 | 0·25 | 0·50 | 0·50 | 0·50 | 0·50 | 1·00 | 0·25 | 0·50 | 0·50 | 1·00 |
| Penicillin | 0·50 | 1·00 | 0·5 | 2·00 | 1·00 | 2·00 | 1·00 | 2·00 | 0·50 | 2·00 | 1·00 | 2·00 |
| Amoxicillin | 1·00 | 2·00 | 1·00 | 4·00 | 1·00 | 2·00 | 1·00 | 4·00 | 0·50 | 2·00 | 1·00 | 4·00 |
| Cefotaxime | 0·25 | 1·00 | 0·25 | 1·00 | 1·00 | 1·00 | 1·00 | 2·00 | 0·50 | 2·00 | 1·00 | 2·00 |
| Serotype 11A, mg/L | ||||||||||||
| Cefixime | 8·00 | 8·00 | 16·00 | 16·00 | 16·00 | 16·00 | 16·00 | 16·00 | 16·00 | 16·00 | 16·00 | 16·00 |
| Cefpodoxime | 0·50 | 0·50 | 2·00 | 2·00 | 2·00 | 2·00 | 2·00 | 2·00 | 2·00 | 2·00 | 2·00 | 2·00 |
| Cefditoren | 0·25 | 0·50 | 0·50 | 0·50 | 0·50 | 0·50 | 0·50 | 0·50 | 0·50 | 1·00 | 0·50 | 0·50 |
| Penicillin | 0·25 | 0·25 | 2·00 | 2·00 | 2·00 | 4·00 | 2·00 | 2·00 | 2·00 | 2·00 | 2·00 | 4·00 |
| Amoxicillin | 0·50 | 0·50 | 4·00 | 8·00 | 4·00 | 8·00 | 4·00 | 4·00 | 4·00 | 8·00 | 4·00 | 8·00 |
| Cefotaxime | 0·25 | 0·25 | 1·00 | 1·00 | 1·00 | 2·00 | 1·00 | 1·00 | 1·00 | 2·00 | 1·00 | 2·00 |
| Serotype 24F, mg/L | ||||||||||||
| Cefixime | 4·00 | 4·00 | 4·00 | 4·00 | 4·00 | 4·00 | 4·00 | 4·00 | 4·00 | 4·00 | 4·00 | 4·00 |
| Cefpodoxime | 0·25 | 0·50 | 0·25 | 0·25 | 0·25 | 0·25 | 0·25 | 0·50 | 0·25 | 0·25 | 0·25 | 0·25 |
| Cefditoren | 0·12 | 0·12 | 0·12 | 0·12 | 0·12 | 0·12 | 0·12 | 0·25 | 0·12 | 0·25 | 0·12 | 0·25 |
| Penicillin | 0·50 | 0·50 | 0·50 | 1·00 | 0·50 | 1·00 | 0·50 | 0·50 | 0·50 | 1·00 | 0·50 | 1·00 |
| Amoxicillin | 0·12 | 0·25 | 0·12 | 0·50 | 0·06 | 0·12 | 0·06 | 0·12 | 0·06 | 0·12 | 0·06 | 0·12 |
| Cefotaxime | 0·12 | 0·25 | 0·25 | 0·25 | 0·25 | 0·50 | 0·25 | 0·25 | 0·25 | 0·50 | 0·25 | 0·25 |
| Serotype 23B, mg/L | ||||||||||||
| Cefixime | 4·00 | 4·00 | 2·00 | 2·00 | 2·00 | 4·00 | 2·00 | 2·00 | 2·00 | 4·00 | 2·00 | 2·00 |
| Cefpodoxime | 0·25 | 0·25 | 0·12 | 0·12 | 0·12 | 1·00 | 0·12 | 0·25 | 0·12 | 1·00 | 0·12 | 0·12 |
| Cefditoren | 0·12 | 0·12 | 0·06 | 0·06 | 0·06 | 0·25 | 0·06 | 0·12 | 0·06 | 0·25 | 0·06 | 0·06 |
| Penicillin | 0·25 | 0·25 | 0·12 | 0·25 | 0·25 | 0·25 | 0·25 | 0·25 | 0·25 | 0·50 | 0·25 | 0·50 |
| Amoxicillin | 0·12 | 0·12 | 0·12 | 0·25 | 0·06 | 0·25 | 0·06 | 0·06 | 0·06 | 0·50 | 0·06 | 0·12 |
| Cefotaxime | 0·12 | 0·12 | 0·06 | 0·25 | 0·12 | 0·25 | 0·12 | 0·25 | 0·12 | 0·50 | 0·12 | 0·25 |
MIC=minimum inhibitory concentration.
For penicillin and amoxicillin, the three most frequent PCV13 serotypes (19A, 14, and 19F) had higher MIC50 or MIC90 values than the non-PCV13 serotypes 24F and 23B. However, serotype 11A (which is not included in PCV13 but is included in PCV20 and PPV23) was the serotype with the highest MIC50 or MIC90 values since 2008, being even higher than the three PCV13 serotypes studied (table). In terms of antibiotic resistance and SARS-CoV-2, we found an increase in the MIC90 values to penicillin for serotype 11A, which changed the interpretation from susceptible with increased exposure to resistant. Hence, the MIC90 value for serotype 11A increased from 2 μg/mL in 2016–19 to 4 μg/mL in 2020 (table).
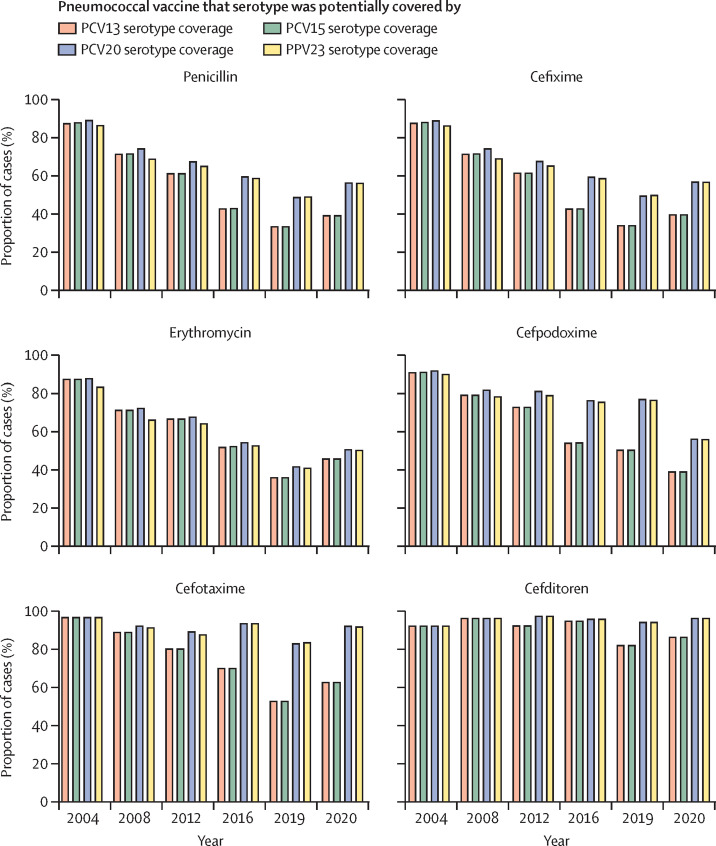
In this study, we explored the proportion of pneumococcal disease caused by strains with reduced susceptibility to different antibiotics that are potentially covered by different PCVs and PPV23 (figure 4 ). During the late PCV7 and early PCV13 periods (2008–12), the majority of pneumococcal cases associated with reduced susceptibility were caused by PCV13 serotypes (figure 4). Our results suggest that in comparison with PCV13 or PCV15, PCV20 would increase by up to 30% the potential coverage of cases by strains with reduced susceptibility to β-lactams (figure 4). Overall, the use of PPV23, despite containing three more serotypes than PCV20, offered similar protection against resistant strains (figure 4).
Figure 4.
Proportion of cases of pneumococcal disease caused by strains potentially covered by PCVs and PPV23 but which had reduced susceptibility to different antibiotics, 2004–20
PCV=pneumococcal conjugate vaccine. PPV=pneumococcal polysaccharide vaccine.
From the antibiotic perspective, for cefditoren and cefotaxime (which were the cephalosporins showing the best antimicrobial activity), the use of PCV20 would prevent more than 92% of all cases produced by pneumococcal strains that have reduced susceptibility to those antibiotics (figure 4).
Discussion
Antibiotic treatment with β-lactam antibiotics, including the use of third-generation cephalosporins, is one the first options for the management of pneumococcal infections.13, 14 A major threat in public health is the rise of resistant strains that can increase mortality rates by reducing the efficacy of antibiotic treatment.15 The use of PCVs in children and adults has been shown to be an effective intervention to control the burden of invasive and non-invasive disease and a great measure to reduce the effect of antimicrobial resistance.16, 17
In this study, we analysed the evolution of antimicrobial resistance in S pneumoniae in strains not susceptible to penicillin, including the contribution of different PCVs, to ameliorate the problem of antibiotic resistance. One of the main mechanisms for reduced susceptibility to β-lactam antibiotics (including penicillins and cephalosporins) is the mutation in penicillin-binding proteins.18 Our results showed that the cephalosporin with the highest activity in terms of MIC50 or MIC90 values was cefditoren, which showed the greatest proportion (>80%) of susceptible strains during 2004–20. These results are in agreement with previous reports19, 20 that suggested a marked activity of this cephalosporin against penicillin-resistant pneumococcal strains, because of its high affinity to penicillin-binding protein 2X (PBP2X). Owing to its high antimicrobial activity, the proportion of strains resistant to cefditoren in our study was extremely low (<0·4%), despite the long-term use of this oral antibiotic in Spain since 2004.21 These results were substantially different to those for the other oral cephalosporins tested, which had far higher proportions of resistant strains (68% for cefixime and 50% for cefpodoxime). Cefditoren, followed by cefotaxime, were the cephalosporins with the highest activity against serotypes during the study period. This activity is important against respiratory infections, because cefditoren has a similar bacterial spectrum to cefotaxime or ceftriaxone, and can be used as an oral treatment against community-acquired bacterial pneumonia in patients who have not been hospitalised or after intravenous treatment with parenteral cephalosporins.21, 22, 23 Another benefit of using cefditoren is that, because its intrinsic activity is higher than for other cephalosporins, it could help to reduce the length of hospital stay and thereby the risk of hospital-acquired infection by multidrug-resistant strains.24 Levofloxacin was one of the antibiotics with the lowest proportion of resistant strains. This result was in agreement with a 2016 surveillance study6 that compared different countries, which found that levofloxacin was one of the most active agents against multidrug-resistant pneumococcal strains.6
Our data show the effectiveness of the different PCVs for controlling the dissemination of pneumococcal-resistant strains, and suggest that the use of these vaccines in national immunisation schedules is a cost-effective countermeasure to antibiotic resistance.16 In the pre-PCV period, the majority of cases were caused by serotypes included in the vaccines and were associated with multidrug resistance.8, 15, 25 Our results reinforce this relationship, but also show a clear benefit in reducing vaccine-serotypes after the use of PCV7 and PCV13, although for serotype 19A, a situation of stability was observed in the past 5 years. This stability is intriguing, because PCV13 was included in the national paediatric immunisation schedule, which has had high coverage rates since 2016 and which led to the expectation of a more profound effect. This plateau perhaps therefore indicates the maximum benefit that can be achieved after several years of use. However, the emergence in our study of non-vaccine serotypes that harbour antibiotic resistance shows that this issue is a global threat, given that many other countries have reported similar replacement in pneumococcal serotypes and lineages.5, 25, 26, 27
The emergence of penicillin-resistant strains of serotype 11A is concerning from a pathogenesis perspective. This serotype contains a particular clone (ST652111A) that has become one of the most prevalent among serotype 11A, with an increased ability to produce biofilms and invasive disease by very efficiently diverting the host immune response.15 Hence, the profound potential of this serotype to produce infection might explain why serotype 11A was the serotype with the second highest fatality rate in a lethality study.28 Another non-PCV13 serotype that has emerged is serotype 24F. This serotype is also alarming, because it displays resistance to penicillin and erythromycin, and its prevalence in the paediatric and adult population is increasing in various countries.5
A limitation of our study is that from each year we selected around 500 strains with penicillin susceptibility with increased exposure or resistance, rather than all pneumococcal strains, and therefore our results might underestimate the potential effect of PCVs in reducing the burden of disease caused by resistant serotypes. We did not include paediatric strains and, although the majority of serotypes affecting children are similar to those in adults,5 our results might not be generalisable to children.
During the first year of the COVID-19 pandemic, generic use of antibiotics to avoid co-infections with bacterial pathogens might explain the increased proportion of pneumococcal strains resistant to different antimicrobial drugs.29 This idea is consistent with a clinical trial30 published in 2021 that advised against the routine use of azithromycin in people with suspected COVID-19 in the community because it might exacerbate the antimicrobial resistance problem. The increased resistance to penicillin for serotype 11A in Spain during the COVID-19 pandemic is worrying and deserves further attention, because it changes the consideration from a serotype with reduced susceptibility to a serotype that is resistant, according to MIC90 values in 2016–2019 and 2020.
The introduction of newer PCVs with a broader spectrum of covered serotypes might help to resolve the problem of non-PCV13 serotypes with antibiotic resistance. The difference in the effect of PCV15 compared with PCV13 was minimal in terms of increased coverage against non-susceptible strains to antibiotics, whereas PCV20 markedly enhanced the potential coverage against non-susceptible strains, as PCV20 could prevent 92% of strains not susceptible to cefotaxime.
In the context of using PCVs that have a higher spectrum, such as PCV20 (with the potential risk of replacement by non-vaccine serotypes after their implementation), the antibiotics with the highest activity against non-PCV20 strains were cefotaxime as a parenteral option, and cefditoren as an oral option. This finding might be important in helping to avoid the selection of resistant strains after massive use of this vaccine in the general population. For resistance to erythromycin, the potential coverage of PCV20 and PPV23 is more limited than for penicillin because they did not prevent cases caused by serotype 24F, which was the most frequent cause of infection associated with erythromycin resistance.
Overall, our results support the potential of cefditoren as an oral administration option for pneumococcal disease, based on its high antimicrobial activity, and highlight the increase in non-PCV13 serotypes, especially serotype 11A, which can be further prevented by the use of PCV20 or PPV23.
For EUCAST clinical breakpoints see https://www.eucast.org/clinical_breakpoints/
Data sharing
All data requests should be submitted to MD (miridome@ucm.es) or JY (jyuste@isciii.es). Requests will be assessed for scientific rigour before being granted and a data-sharing agreement might be required.
Declaration of interests
JY received grants from MSD-USA (Merck Investigator Studies Program), and Pfizer, outside of this work. JY participated in advisory boards organised by MSD and Pfizer. MG and PC are members of the Scientific Department, Meiji Pharma Spain. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
We thank Dolores Vicioso and Asunción Fenoll for assistance with the epidemiological surveillance. This work was supported by the Spanish Ministry of Science and Innovation (grant PID2020–119298RB-I00), Meiji Pharma Spain (grant MVP 119/20), and internal funding from Instituto de Salud Carlos III.
Contributors
JY was responsible for the management of the epidemiological surveillance data. JY wrote the first draft of the paper. ML, JS, BLR, IDR, CPG, DL, MG, PC, FGC, MD, and JY provided technical support for the study. MG, PC, MD, and JY contributed to the study conception, design, data analysis, and interpretation. All authors contributed to the review of the different drafts, and approved all versions of the manuscript. All authors had full access to all the data in the study and had final responsibility for the decision to submit for publication. JS, MD, and JY accessed and verified all the data.
Supplementary Materials
References
- 1.Aliberti S, Dela Cruz CS, Amati F, Sotgiu G, Restrepo MI. Community-acquired pneumonia. Lancet. 2021;398:906–919. doi: 10.1016/S0140-6736(21)00630-9. [DOI] [PubMed] [Google Scholar]
- 2.GBD 2016 Lower Respiratory Infections Collaborators Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018;18:1191–1210. doi: 10.1016/S1473-3099(18)30310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonten MJ, Huijts SM, Bolkenbaas M, et al. Polysaccharide conjugate vaccine against pneumococcal pneumonia in adults. N Engl J Med. 2015;372:1114–1125. doi: 10.1056/NEJMoa1408544. [DOI] [PubMed] [Google Scholar]
- 4.Torres A, Menéndez R, España PP, et al. The evolution and distribution of pneumococcal serotypes in adults hospitalized with community-acquired pneumonia in Spain using a serotype-specific urinary antigen detection test: the CAPA Study, 2011–2018. Clin Infect Dis. 2021;73:1075–1085. doi: 10.1093/cid/ciab307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Miguel S, Domenech M, González-Camacho F, et al. Nationwide trends of invasive pneumococcal disease in Spain from 2009 through 2019 in children and adults during the pneumococcal conjugate vaccine era. Clin Infect Dis. 2021;73:e3778–e3787. doi: 10.1093/cid/ciaa1483. [DOI] [PubMed] [Google Scholar]
- 6.Sader HS, Mendes RE, Le J, Denys G, Flamm RK, Jones RN. Antimicrobial susceptibility of Streptococcus pneumoniae from North America, Europe, Latin America, and the Asia-Pacific Region: results from 20 years of the SENTRY antimicrobial surveillance program (1997–2016) Open Forum Infect Dis. 2019;6(suppl 1):S14–S23. doi: 10.1093/ofid/ofy263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sempere J, de Miguel S, González-Camacho F, Yuste J, Domenech M. Clinical relevance and molecular pathogenesis of the emerging serotypes 22F and 33F of Streptococcus pneumoniae in Spain. Front Microbiol. 2020;11:309. doi: 10.3389/fmicb.2020.00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fenoll A, Granizo JJ, Giménez MJ, Yuste J, Aguilar L. Secular trends (1990–2013) in serotypes and associated non-susceptibility of S. pneumoniae isolates causing invasive disease in the pre-/post-era of pneumococcal conjugate vaccines in Spanish regions without universal paediatric pneumococcal vaccination. Vaccine. 2015;33:5691–5699. doi: 10.1016/j.vaccine.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Rosen JB, Thomas AR, Lexau CA, et al. Geographic variation in invasive pneumococcal disease following pneumococcal conjugate vaccine introduction in the United States. Clin Infect Dis. 2011;53:137–143. doi: 10.1093/cid/cir326. [DOI] [PubMed] [Google Scholar]
- 10.Lewnard JA, Bruxvoort KJ, Fischer H, et al. Prevention of COVID-19 among older adults receiving pneumococcal conjugate vaccine suggests interactions between Streptococcus pneumoniae and SARS-CoV-2 in the respiratory tract. J Infect Dis. 2021 doi: 10.1093/infdis/jiab128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitsi E, Reiné J, Urban BC, et al. Streptococcus pneumoniae colonization associates with impaired adaptive immune responses against SARS-CoV-2. J Clin Invest. 2022;132 doi: 10.1172/JCI157124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bornstein DL, Schiffman G, Bernheimer HP, Austrian R. Capsulation of pneumococcus with soluble C-like (Cs) polysaccharide. I. Biological and genetic properties of Cs pneumococcal strains. J Exp Med. 1968;128:1385–1400. doi: 10.1084/jem.128.6.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mandell LA, Wunderink RG, Anzueto A, et al. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis. 2007;44(suppl 2):S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tomczyk S, Jain S, Bramley AM, et al. Antibiotic prescribing for adults hospitalized in the etiology of pneumonia in the community study. Open Forum Infect Dis. 2017;4 doi: 10.1093/ofid/ofx088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aguinagalde L, Corsini B, Domenech A, et al. Emergence of amoxicillin-resistant variants of Spain9v-ST156 pneumococci expressing serotype 11A correlates with their ability to evade the host immune response. PLoS One. 2015;10 doi: 10.1371/journal.pone.0137565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Atkins KE, Flasche S. Vaccination to reduce antimicrobial resistance. Lancet Glob Health. 2018;6:e252. doi: 10.1016/S2214-109X(18)30043-3. [DOI] [PubMed] [Google Scholar]
- 17.Andrejko K, Ratnasiri B, Hausdorff WP, Laxminarayan R, Lewnard JA. Antimicrobial resistance in paediatric Streptococcus pneumoniae isolates amid global implementation of pneumococcal conjugate vaccines: a systematic review and meta-regression analysis. Lancet Microbe. 2021;2:e450–e460. doi: 10.1016/S2666-5247(21)00064-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagai K, Davies TA, Jacobs MR, Appelbaum PC. Effects of amino acid alterations in penicillin-binding proteins (PBPs) 1a, 2b, and 2x on PBP affinities of penicillin, ampicillin, amoxicillin, cefditoren, cefuroxime, cefprozil, and cefaclor in 18 clinical isolates of penicillin-susceptible, -intermediate, and -resistant pneumococci. Antimicrob Agents Chemother. 2002;46:1273–1280. doi: 10.1128/AAC.46.5.1273-1280.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamada M, Watanabe T, Miyara T, et al. Crystal structure of cefditoren complexed with Streptococcus pneumoniae penicillin-binding protein 2X: structural basis for its high antimicrobial activity. Antimicrob Agents Chemother. 2007;51:3902–3907. doi: 10.1128/AAC.00743-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang Q, Xu Y, Chen M, et al. In vitro activity of cefditoren and other comparators against Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis causing community-acquired respiratory tract infections in China. Diagn Microbiol Infect Dis. 2012;73:187–191. doi: 10.1016/j.diagmicrobio.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 21.Granizo JJ, Giménez MJ, Barberán J, Coronel P, Gimeno M, Aguilar L. The efficacy of cefditoren pivoxil in the treatment of lower respiratory tract infections, with a focus on the per-pathogen bacteriologic response in infections caused by Streptococcus pneumoniae and Haemophilus influenzae: a pooled analysis of seven clinical trials. Clin Ther. 2006;28:2061–2069. doi: 10.1016/j.clinthera.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 22.Di Marco F, Braido F, Santus P, Scichilone N, Blasi F. The role of cefditoren in the treatment of lower community-acquired respiratory tract infections (LRTIs): from bacterial eradication to reduced lung inflammation and epithelial damage. Eur Rev Med Pharmacol Sci. 2014;18:321–332. [PubMed] [Google Scholar]
- 23.Monmaturapoj T, Montakantikul P, Mootsikapun P, Tragulpiankit P. A prospective, randomized, double dummy, placebo-controlled trial of oral cefditoren pivoxil 400 mg once daily as switch therapy after intravenous ceftriaxone in the treatment of acute pyelonephritis. Int J Infect Dis. 2012;16:e843–e849. doi: 10.1016/j.ijid.2012.07.009. [DOI] [PubMed] [Google Scholar]
- 24.Blasi F, Concia E, Del Prato B, et al. The most appropriate therapeutic strategy for acute lower respiratory tract infections: a Delphi-based approach. J Chemother. 2017;29:274–286. doi: 10.1080/1120009X.2017.1291467. [DOI] [PubMed] [Google Scholar]
- 25.Cassiolato AP, Almeida SCG, Andrade AL, Minamisava R, Brandileone MCC. Expansion of the multidrug-resistant clonal complex 320 among invasive Streptococcus pneumoniae serotype 19A after the introduction of a ten-valent pneumococcal conjugate vaccine in Brazil. PLoS One. 2018;13 doi: 10.1371/journal.pone.0208211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lo SW, Gladstone RA, van Tonder AJ, et al. Pneumococcal lineages associated with serotype replacement and antibiotic resistance in childhood invasive pneumococcal disease in the post-PCV13 era: an international whole-genome sequencing study. Lancet Infect Dis. 2019;19:759–769. doi: 10.1016/S1473-3099(19)30297-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gladstone RA, Lo SW, Lees JA, et al. International genomic definition of pneumococcal lineages, to contextualise disease, antibiotic resistance and vaccine impact. EBioMedicine. 2019;43:338–346. doi: 10.1016/j.ebiom.2019.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.De Miguel S, Latasa P, Yuste J, et al. Age-dependent serotype-associated case-fatality rate in invasive pneumococcal disease in the autonomous community of Madrid between 2007 and 2020. Microorganisms. 2021;9 doi: 10.3390/microorganisms9112286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Langford BJ, So M, Raybardhan S, et al. Antibiotic prescribing in patients with COVID-19: rapid review and meta-analysis. Clin Microbiol Infect. 2021;27:520–531. doi: 10.1016/j.cmi.2020.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.PRINCIPLE Trial Collaborative Group Azithromycin for community treatment of suspected COVID-19 in people at increased risk of an adverse clinical course in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial. Lancet. 2021;397:1063–1074. doi: 10.1016/S0140-6736(21)00461-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data requests should be submitted to MD (miridome@ucm.es) or JY (jyuste@isciii.es). Requests will be assessed for scientific rigour before being granted and a data-sharing agreement might be required.