ABSTRACT
Background
Blood pressure in childhood tracks into later life. Vitamin D status in adults is associated with blood pressure, but the impact of vitamin D status in pregnancy and childhood on blood pressure still needs investigation.
Objective
We investigated whether fetal rather than current vitamin D status is associated with blood pressure in children.
Methods
In a prospective observational study within the population-based Odense Child Cohort (OCC), we examined serum 25-hydroxyvitamin D2+3 [s-25(OH)D] in early and late pregnancy, cord blood, and at 5 y age, and the associations with systolic and diastolic blood pressure (SBP/DBP) in the 5-y-old children (n = 1,677). Multiple regression models were adjusted for maternal country of origin, parity, smoking during pregnancy, 5-y height, and weight. Two-stage mixed effect modeling was performed, integrating all s-25(OH)D data from pregnancy and cord blood.
Results
The median (IQR) s-25(OH)D in early pregnancy, late pregnancy, the umbilical cord, and at 5 y was 65.5 (50.7–78.5), 78.5 (60.3– 95.8), 45.4 (31.1– 60.7), and 71.9 (54.6– 86.5) nmol/L, respectively. The mean ±SD 5-y SBP/DBP was 101.0/63.8 (7.1/5.9) mmHg. In adjusted analyses, a 10 nmol/L increase of s-25(OH)D in early pregnancy associated with a 0.3/0.2 mmHg lower SBP/DBP at 5 y (P < 0.05). Optimal s-25(OH)D (>75 nmol/L) in early pregnancy was associated with lower 5-y SBP and DBP, β (95% CI) −1.45 (−2.6, −0.3), and −0.97 (−1.9, −0.1), compared with reference s-25(OH)D (50-74.9 nmol/L). Two-stage analysis combining early pregnancy, late pregnancy, and cord s-25(OH)D data showed an inverse association with 5-y SBP and DBP for boys (P < 0.025) with significant sex-difference for DBP (Pinteraction = 0.004). No associations were found between s-25(OH)D and 5-y BP above the 90th percentile.
Conclusion
Early pregnancy s-25(OH)D concentrations, especially >75 nmol/L, were inversely associated with 5-y blood pressure in the offspring. A novel identified protective effect of optimal vitamin D levels in early pregnancy on offspring BP is suggested.
Keywords: vitamin D status, 25(OH)D, pregnancy, fetal programming, children, blood pressure, cardio-metabolic health, cohort
Introduction
High blood pressure (BP) is the most important modifiable risk factor for cardiovascular events and overall disease burden in adults (1). Much interest has been given to vitamin D supplementation as a potential prevention measure against high BP or hypertension. Recent systematic reviews with meta-analysis of randomized controlled trials (RCTs) do not, however, support an effect of supplementation with vitamin D alone (2, 3) or calcium and vitamin D combined (4), on systolic BP (SBP) or diastolic BP ( DBP). One exception was seen for individuals with pre-existing cardiovascular disease, who had a 1.31 mmHg reduced DBP after vitamin D supplementation (5).
On the other hand, baseline s-25-hydroxyvitamin D [s-25(OH)D] was inversely associated with risk of hypertension in recent meta-analyses of observational studies (3, 6), and a Mendelian randomization study provided evidence for a protective effect of higher vitamin D status on SBP, DBP, and hypertension (7). Several biological mechanisms have been proposed for a protective effect of vitamin D on hypertension, which has been supported by animal studies (8).
Elevated BP in children is a strong predictor of hypertension later in life (9, 10). Although no effect of vitamin D supplementation on BP was seen in a meta-analysis of RCTs in children and adolescents (11), higher vitamin D status is associated with lower BP in children in several observational studies (12–16). Furthermore, an association between vitamin D status [s-25(OH)D] in pregnancy or cord blood and offspring BP has been suggested in some (17–21), but not all studies (22, 23).
Increased BP and hypertension in adulthood may already be determined during the fetal period (24, 25). In humans, nephrogenesis begins in week 5 of pregnancy and impaired nephrogenesis results in reduced nephron numbers, which together with altered renin-angiotensin-aldosterone activity, glucocorticoid excess, altered tubular handling of sodium ions, and inappropriate activation of the endothelin system may lead to subsequent higher BP.
Vitamin D deficiency in pregnancy affects nephrons, glomeruli, renin, and endothelial relaxation, and increases SBP and DBP in animal studies (26–29). A specific vulnerable time window for hypovitaminosis D in the human fetus is not known, but early pregnancy may be the most vulnerable period for the impact of malnutrition on nephrogenesis in this period (30).
Hypovitaminosis D, defined as s-25(OH)D <50 nmol/L, is still very common in pregnant women especially at northern latitudes (31, 32), as also seen in our previous studies from the Odense Child Cohort (OCC) in Denmark, despite recommendations of vitamin D supplementation of 10 µg/d during pregnancy (33, 34). Previous association studies have not had the chance to investigate s-25(OH)D associations from several time points in early life in relation to BP.
In the present study, we aimed to investigate whether s-25(OH)D concentrations in early and late pregnancy, in cord blood, and at 5 y of age were associated with BP in 5-y-old children, hypothesizing early pregnancy to be the most vulnerable exposure time.
Subjects and methods
Design and study population
The study was a part of the OCC, an ongoing population-based prospective, observational mother–child cohort from early pregnancy onward. Newly pregnant women with residence in Odense, Denmark, were invited to participate between 1 January 2010 and 31 December 2012. The cohort included 2,875 pregnant women. A detailed description of the OCC has been given elsewhere (35).
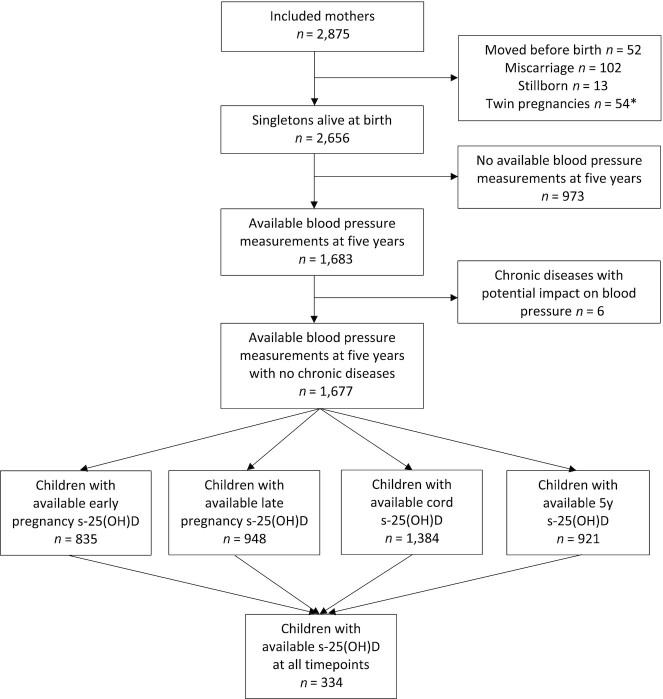
In the present study, exclusion criteria were miscarriage, stillbirth, migration from the study region, and chronic diseases with risk of hypo- or hypertension (e.g., major congenital heart disease or chronic renal insufficiency). Furthermore, only singletons with information on BP at the age of 5 y and s-25(OH)D determined at either one, some, or all the time points: early and late pregnancy, in cord blood, and at 5 y, were included (Figure 1).
FIGURE 1.
Participant inclusion flowchart. *Some participants were excluded due to >1 criterion. s-25(OH)D, serum 25-hydroxyvitamin D2+D3.
To best reflect a general population, mothers with preeclampsia (n = 114) or gestational hypertension (n = 67) were not excluded. Likewise, we included 64 preterm infants (GA median: 35 wk 5 d; range: 27 wk 1 d to 36 wk 6 d) and 14 term neonates with low birth weight (mean: 2,334 g; range: 1,800–2,495 g).
Vitamin D status
The vitamin D status was based on s-25(OH) D concentrations, considered to be the best marker of vitamin D status (36), and analyzed using gold standard HPLC-MS, calibrated against National Institute of Standards and Technology standard 972 as previously described in detail (33). S-25(OH)D was given as the sum of s-25(OH) D2 and s-25(OH) D3.
Blood samples were drawn during early pregnancy, median (IQR) gestational age 12.1 (10–15) wk; late pregnancy, 29 (28–30) wk, from the umbilical cord; and at child age 5.0 (5.0–5.1) y.
Blood pressure measurements
At the child's 5-y visit, SBP and DBP were measured with Welch Allyn vital signs on the left arm twice with cuffs of appropriate sizes and with the child in a sitting position and the arm resting down the side, with a 1-min rest before the measurement (37). All measures were performed by trained professionals.
Covariates
Information about covariates was obtained through medical records, self-reported data, questionnaires, and physical examination of the children performed by OCC staff, blinded for s-25(OH)D, at 3 and 18 mo, and 3 and 5 y. The Municipality of Odense provided information about the mothers’ birth country and parental ethnicity (Danish/other Western or non-Western country of origin). Data on parity, maternal prepregnancy BMI, and smoking during pregnancy were retrieved from the journal report at the first antenatal visit. Maternal BP in the first, second, and third trimester of pregnancy was obtained as previously reported (38). Through questionnaires answered during pregnancy and 3 mo after birth, information was obtained about gestational weight gain, use of vitamin D supplement during pregnancy (<10 mg/daily or ≥10 mg/daily), maternal skin type on modified Fitzpatrick's Scale (I to VI) (39), sun exposure during pregnancy (never/rarely, sometimes, often, or most of the time), and parental education level (high school or less, high school 1–3 or ≥4 y). Parental-reported information about child vitamin D supplementation (µg/d) and duration of exclusive breastfeeding (wk) were collected in a later questionnaire, ∼18 mo after birth. Information about child skin type on the Fitzpatrick's scale was drawn from questionnaires completed by parents when their child was 3 y old.
The children's skin types were merged into 3 groups: I/II, III, and IV/V/VI, due to few participants in group I, V, and VI.
Information about the children, including sex and gestational age at birth (in days), was collected from medical files. Maternal age was calculated at the time of birth. The time of blood sampling was categorized as high or low vitamin D season (May–October or November–April). At the 5-y examination, child height (to the nearest centimeter) was measured with a stadiometer and child weight (to the nearest 0.1 kg) without or with minimal clothing was measured by trained staff professionals at OCC on a digital weight scale.
Statistical analysis
Numerical data were presented as mean ± SD or median and IQR, where appropriate. BP was used as a continuous variable and was also dichotomized at the ≥90th compared with the <90th percentile of our own data set. S-25(OH)D was used as a continuous variable and categorized according to quartiles and the routine cutoffs <25, 25–49.9, 50–74.9, and ≥75 nmol/L using the 1st quartile and 50–74.9 nmol/L as references. Differences between participant characteristics in quartiles of early pregnancy s-25(OH)D were examined using an ANOVA or Kruskal–Wallis test for continuous variables and chi-square test for categorical variables. A Kruskal–Wallis test was also used to evaluate sex differences in s-25(OH)D.
Density plots with kernel distribution for s-25(OH)D at each time point and locally weighted scatterplot smoothing (lowess) for SBP/DBP compared with s-25(OH)D split by sex were performed for visual inspection. Univariate and multiple linear regression analyses were applied to test the associations between the measures of s-25(OH)D at the 4 different time points and 5-y BP. Moreover, the association of the s-25(OH)D samples at the 4 time points with BP ≥90th percentile for the cohort was examined using multiple logistic regression models. The association between early pregnancy s-25(OH)D and 5-y SBP was chosen as the primary association and did not change throughout the course of the research.
Missing covariate data were handled by exclusion of the participants with missing data from the adjusted analysis. Analyses were performed in the whole group as well as stratified by sex. Potential effect modification of sex on the association between vitamin D status and BP was examined by including an interaction term in the final adjusted models.
A two-stage model was applied to utilize all available s-25(OH)D data from early pregnancy and late pregnancy and cord blood in the analysis of a combined association to 5-y BP. The first stage applied a mixed effects linear regression adjusting for time point and with residual variance stratified by time point to determine each child individual overall level of s-25(OH)D as a random intercept. In a second stage, this determined level was applied as exposure in a linear regression with BP at 5 y as outcome adjusted for covariates. To consider the combined uncertainty in both steps, CIs and P values were determined by bootstrapping with 1,000 repetitions.
Analyses were conducted using Stata 15.0 software (StataCorp).
Model assumptions were checked by visual inspection of the distribution of the studentized residuals in a normal quantile-quantile plot. Furthermore, logistic regression models were tested by Pearson's goodness-of-fit test.
Two-sided P values <0.05 were considered statistically significant. No corrections for multiple testing were applied in the main analyses, as significant associations in the primary analysis (early pregnancy s-25(OH)D and 5-y BP) were supported by several similar findings. As a sensitivity analysis, however, we applied the Bonferroni adjusted P value <0.025 to correct for two outcomes (SBP and DBP).
Our study was a priori powered to detect a difference in SBP of 0.33 mmHg and DBP 0.27 mmHg for every 10 nmol/L difference in s-25(OH)D given n = 800, alfa = 0.05, beta = 0.20, early pregnancy s-25(OH)D ±SD 21.53 nmol/L, SBP ±SD = 7.10 mmHg, and DBP ±SD 5.81 mmHg.
Differences between participants and nonparticipants were assessed using Student's t-test or Mann-Whitney for continuous Gaussian or non-Gaussian data, respectively, and chi-square tests were applied for categorical variables. Nonparticipants were defined as included in OCC, but not participating in this study due to the exclusion criteria.
Analysis not prespecified were considered exploratory.
Ethics
The study was approved by the Regional Scientific Ethical Committee of Southern Denmark (no. S‐20090130) and the Danish Data protection Board (application no. 13/14088). All women willing to participate gave informed written consent at enrollment. The study was carried out in accordance with the Helsinki Declaration II and reported according to STROBE guidelines for observational studies (40).
Results
Study population, exposure, and outcome
The present study included 1,677 mother-child pairs with data on 5-y BP and s-25(OH)D at any of the 4 time points. In early pregnancy, late pregnancy, cord blood, and at 5-y, hypovitaminosis D [s-25(OH)D <50 nmol/L] was found in 24.0%, 15.6%, 58.5%, and 19.7%, respectively. The medians [IQR] of s-25(OH)D at these time periods were 65.5 (50.7; 78.5), 78.5 (60.3; 95.8), 45.4 (31.1; 60.7) and 71.9 (54.6; 86.5) nmol/L, respectively. No sex difference in s-25(OH)D concentrations was found in pregnancy or cord samples, Supplementary Figure 1. At 5 y, boys had higher s-25(OH)D than girls, 74.2 (56.0; 88.1) nmol/L compared with 70.0 (53.4; 84.6) nmol/L, P = 0.04.
Compared with participants in this study, nonparticipant mothers within OCC were younger, darker skinned, and more likely to smoke during pregnancy and be of non-Western ethnicity. Nonparticipant children were more likely to be born earlier, have lower birthweight, not to be the first child, have darker skin, and be exclusively breastfeed for a shorter period (Supplementary Table 1).
Participant characteristics according to quartiles of early pregnancy s-25(OH)D are presented in Table 1. Mothers with s-25(OH)D in the 1st quartile (Q1; <50.7 nmol/L) had higher prepregnancy BMI, higher parity, less vitamin D supplementation during pregnancy, were more often of non-Western ethnicity, and were more likely to smoke during pregnancy compared with mothers with s-25(OH)D in higher quartiles. Early pregnancy blood sample season in May-October and lighter skin type of the child were significantly associated with higher quartiles of early pregnancy s-25(OH)D.
TABLE 1.
Selected population characteristics by quartiles of early pregnancy s-25(OH)D1
| All quartiles of s-25(OH)D in early pregnancy, nmol/L | |||||||
|---|---|---|---|---|---|---|---|
| n | ≥50.7 | <50.7 | 50.7–65.5 | >65.6 to 78.5 | >78.5 | P value | |
| Maternal characteristics | |||||||
| Age, y | 835 | 30.3 (± 4.4) | 30.7 (± 4.6) | 30.6 (± 4.3) | 30.1 (± 4.4) | 29.8 (± 4.5) | 0.125 |
| Pregestational BMI | 835 | 23.6 [21.5; 26.2] | 24.5 [22.2; 27.5] | 23.7 [21.7; 25.9] | 23.4 [21.4; 26.0] | 22.8 [21.2; 25.5] | <0.001 |
| Smoking2, n (%) | 835 | 36 (4.3) | 15 (7.2) | 6 (2.9) | 4 (1.9) | 11 (5.3) | 0.035 |
| Alcohol consumption2, n (%) | 626 | 47 (7.5) | 12 (8.3) | 12 (7.6) | 11 (6.9) | 12 (7.3) | 0.974 |
| Education level | 823 | 0.531 | |||||
| Low, n (%) | 233 (28.3) | 57 (27.8) | 50 (24.3) | 64 (31.2) | 62 (30.0) | ||
| Intermediate, n (%) | 409 (49.7) | 107 (52.2) | 102 (49.5) | 98 (47.8) | 102 (49.3) | ||
| High, n (%) | 181 (22.0) | 41 (20.0) | 54 (26.2) | 43 (21.0) | 43 (20.8) | ||
| Ethnicity | 835 | <0.001 | |||||
| Danish/Western, n (%) | 807 (96.7) | 192 (91.9) | 206 (98.6) | 201 (96.2) | 208 (100.0) | ||
| Non-Western country, n (%) | 28 (3.4) | 17 (8.1) | 3 (1.4) | 8 (3.8) | 0 (0.0) | ||
| Maternal skin type (Fitzpatrick) | 632 | 0.328 | |||||
| I/II, n (%) | 122 (19.3) | 33 (22.5) | 24 (15.2) | 27 (16.8) | 38 (22.9) | ||
| III, n (%) | 387 (61.2) | 88 (59.9) | 93 (58.9) | 107 (66.5) | 99 (59.7) | ||
| IV, n (%) | 117 (18.5) | 25 (17.0) | 38 (24.1) | 26 (16.2) | 28 (16.9) | ||
| V/VI, n (%) | 6 (1.0) | 1 (0.7) | 3 (1.9) | 1 (0.6) | 1 (0.6) | ||
| Parity | 835 | <0.001 | |||||
| 1, n (%) | 473 (56.7) | 88 (42.1) | 129 (61.7) | 125 (59.8) | 131 (56.7) | ||
| ≥2, n (%) | 362 (43.4) | 121 (57.9) | 80 (38.3) | 84 (40.2) | 77 (37.0) | ||
| Vitamin D tablets3, n (%) | 522 | 461 (88.3) | 92 (83.6) | 111 (84.7) | 127 (90.1) | 131 (93.6) | 0.043 |
| Sun exposure2 | 634 | 0.455 | |||||
| Never/rarely, n (%) | 10 (1.6) | 1 (0.7) | 4 (2.5) | 3 (1.9) | 2 (1.2) | ||
| Sometimes, n (%) | 135 (21.3) | 37 (25.2) | 30 (18.9) | 40 (24.7) | 28 (16.9) | ||
| Often, n (%) | 386 (60.9) | 83 (56.5) | 104 (65.4) | 92 (56.8) | 107 (64.5) | ||
| Most of the time, n (%) | 103 (16.3) | 26 (17.7) | 21 (13.2) | 27 (16.7) | 29 (17.5) | ||
| Child characteristics | |||||||
| Skin type (Fitzpatrick) | 688 | 0.012 | |||||
| I/II, n (%) | 361 (52.5) | 84 (45.9) | 94 (55.6) | 98 (55.1) | 85 (53.8) | ||
| III, n (%) | 303 (44.0) | 85 (46.5) | 74 (43.8) | 75 (42.1) | 69 (43.7) | ||
| IV/V/VI, n (%) | 24 (3.5) | 14 (7.7) | 1 (0.6) | 5 (2.8) | 4 (2.5) | ||
| Age in y at examination | 834 | 5.01 [5.0; 5.1] | 5.01 [5.0; 5.0] | 5.02 [5.0; 5.1] | 5.02 [5.0; 5.1] | 5.01 [5.0; 5.1] | 0.031 |
| Early pregnancy blood sample, May–Oct, n (%) | 835 | 396 (47.4) | 74 (36.6) | 101 (46.3) | 108 (49.1) | 113 (58.0) | <0.001 |
| Height at 5 y,cm | 834 | 112.3 [109.2; 115.1] | 112.5 [109.7; 114.9] | 112.4 [109.1; 115.3] | 111.8 [108.8; 115.2] | 112.5 [109.3; 115.2] | 0.681 |
| Weight at 5 y, kg | 819 | 19.1 [17.7; 20.6] | 19.4 [18.2; 20.6] | 18.9 [17.9; 20.5] | 18.7 [17.4; 20.4] | 19.1 [17.7; 20.8] | 0.072 |
| BMI at 5 y | 819 | 15.2 [14.5; 15.9] | 15.4 [14.7; 16.0] | 15.1 [14.5; 15.8] | 15.1 [14.4; 15.8] | 15.1 [14.5; 15.9] | 0.046 |
| SPB at 5 y,mm Hg | 835 | 100.9 (±7.3) | 101.5 (±7.3) | 101.4 (±7.9) | 100.4 (±6.8) | 101.4 (±7.1) | 0.223 |
| DBP at 5 y, mm Hg | 835 | 63.6 (±5.8) | 64.0 (±5.7) | 63.9 (±5.5) | 63.6 (±5.8) | 63.1 (±6.1) | 0.360 |
| GA at birth, d | 835 | 282 [275; 288] | 282 [276; 287] | 281 [274; 288] | 281 [275; 287] | 282 [275; 288] | 0.850 |
| Boys, n (%) | 835 | 440 (52.7) | 109 (52.2) | 99 (47.4) | 112 (53.6) | 120 (57.7) | 0.208 |
| Vaginal birth, n (%) | 835 | 684 (81.9) | 166 (79.4) | 173 (82.8) | 170 (81.3) | 175 (84.1) | 0.633 |
| Duration of exclusive breastfeeding,wk | 743 | 8 [0; 17] | 8 [0; 16] | 10 [0; 18] | 12 [0; 17] | 8 [0; 16] | 0.185 |
| Paternal characteristics | |||||||
| BMI | 493 | 25.0 [23.0; 27.4] | 25.3 [23.5; 28.3] | 24.8 [23.0; 26.8] | 25.3 [22.9; 27.4] | 24.7 [22. 8;26.6] | 0.349 |
Values are presented as numbers [n or n (%)], mean ± SD, or median [IQR]. Sun exposure, education, and skin type were analyzed as categorical variables with reference to no sun exposure, low education, and Fitzpatrick I/II skin type. ANOVA or Kruskal–Wallis test used for continuous variables and chi-square test for categorical variables. DBP, diastolic blood pressure; GA, gestational age; SBP, systolic blood pressure; s-25(OH)D, serum 25-hydroxyvitamin D.
During pregnancy.
Vitamin D supplementation >10 µg/d during pregnancy.
The mean ±SD of 5-y SBP/DBP was 101.0/63.8 (7.1/5.9) mmHg with no sex differences; boys 101.2/63.6 (7.1/5.7), girls 100.8/64.0 (7.2/6.0). Lowess smoothing plots for the unadjusted s-25(OH)D associations to 5-y SBP/DBP are given in Supplementary Figure 2.
Early pregnancy s-25(OH)D associations to blood pressure at 5 y
Early pregnancy s-25(OH)D showed inverse associations with 5-y SBP and DBP in the total cohort for all models, whether 25(OH)D was used as continuous or categorized by clinical cutoffs or quartiles (Tables 2–4 and Supplementary Tables 2–4). Optimal s-25(OH)D (≥75 nmol/L) associated with 1.45 mmHg lower SBP (P = 0.01) and 0.97 mmHg lower DBP (P = 0.04) compared with reference s-25(OH)D (50-74.9 nmol/L). No associations were found between early pregnancy s-25(OH)D and offspring risk of increased BP (BP >90th percentile, Supplementary Table 5).
TABLE 2.
Adjusted multiple linear regression of associations between 5-y SBP/DBP and continuous s-25(OH)D in pregnancy and cord blood1
| Association with s-25(OH)D concentration | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Early pregnancy2 | Late pregnancy2 | Cord blood | |||||||
| n | β (95% CI) | P value | n | β (95% CI) | P value | n | β (95% CI) | P value | |
| All | |||||||||
| SBP | 819 | −0.03 (−0.05, −0.01) | 0.015 | 927 | −0.01 (−0.02, 0.01) | 0.486 | 1351 | −0.00 (−0.02, 0.01) | 0.686 |
| DBP | 819 | −0.02 (−0.04, −0.00) | 0.048 | 927 | −0.00 (−0.02, 0.01) | 0.894 | 1351 | −0.00 (−0.02, 0.01) | 0.568 |
| Girls | |||||||||
| SBP | 389 | −0.04 (−0.08, −0.00) | 0.044 | 443 | 0.01 (−0.02, 0.03) | 0.602 | 638 | 0.01 (−0.02, 0.04) | 0.460 |
| DBP | 389 | −0.01 (−0.04, 0.02) | 0.398 | 443 | 0.01 (−0.01, 0.03) | 0.186 | 638 | 0.01 (−0.01, 0.03) | 0.379 |
| Boys | |||||||||
| SBP | 430 | −0.02 (−0.05, 0.01) | 0.166 | 484 | −0.02 (−0.04, 0.00) | 0.097 | 713 | −0.01 (−0.04, 0.01) | 0.249 |
| DBP | 430 | −0.02 (−0.05, 0.00) | 0.083 | 484 | −0.02 (−0.04, 0.00) | 0.124 | 713 | −0.02 (−0.04, 0.00) | 0.084 |
| Interaction, girls vs. boys | |||||||||
| SBP | 0.445 | 0.163 | 0.905 | ||||||
| DBP | 0.857 | 0.056 | 0.892 | ||||||
Values are results of multiple linear regression. All models adjusted for maternal ethnicity, smoking during pregnancy, height at 5 y, weight at 5 y, and parity. DBP, diastolic blood pressure; GA, gestational age; SBP, systolic blood pressure; vs., versus; s-25(OH)D, serum 25-hydroxyvitamin D.
Early pregnancy, GA <140 d; late pregnancy, GA ≥140 d.
TABLE 4.
Adjusted associations between 5-y SBP/DBP and clinical cutoffs for s-25(OH)D in pregnancy and cord blood1
| Association with s-25(OH)D concentration | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| s-25(OH)D, nmol/L | Early pregnancy | Late pregnancy | Cord blood | ||||||
| All participants | n | β (95% CI) | P value | n | β (95% CI) | P value | n | β (95% CI) | P value |
| SBP | 819 | 927 | 1,351 | ||||||
| <25 | 17 | 1.08 (−2.4, 4.5) | 0.538 | 13 | 1.03 (−2.8, 4.9) | 0.603 | 216 | 1.04 (−0.1, 2.2) | 0.077 |
| 25–49.9 | 177 | −0.39 (−1.7, 0.9) | 0.552 | 129 | −0.42 (−1.9, 1.0) | 0.571 | 571 | 0.60 (−0.3, 1.5) | 0.182 |
| 0–49.9 | 194 | −0.26 (−1.5, 1.0) | 0.681 | 142 | −0.29 (−1.7, 1.1) | 0.686 | 423 | 0.71 (−0.1, 1.5) | 0.090 |
| 50–74.9 | 358 | Reference | 266 | Reference | 141 | Reference | |||
| ≥75 | 267 | −1.45 (−2.6, −0.3) | 0.011 | 519 | −0.45 (−1.5, 0.6) | 0.389 | 80 | 0.85 (−0.5, 2.2) | 0.210 |
| Trend2 | 819 | −0.65 (−01.3, −0.0) | 0.043 | 927 | −0.18 (−0.8, 0.4) | 0.552 | 1,351 | −0.22 (−0.7, 0.2) | 0.305 |
| DBP | 819 | 927 | 1,351 | ||||||
| <25 | 17 | −0.72 (−3.5, 2.1) | 0.611 | 13 | 0.82 (−2.4, 4.0) | 0.616 | 216 | 0.70 (−0.3, 1.7) | 0.152 |
| 25–49.9 | 177 | −0.11 (−1.2, 0.9) | 0.836 | 129 | −0.52 (−1.7, 0.7) | 0.405 | 571 | 0.77 (0.0, 1.5) | 0.039 |
| 0–49.9 | 194 | −0.16 (−1.2, 0.9) | 0.752 | 142 | −0.39 (−1.6, 0.8) | 0.511 | 423 | 0.75 (0.1, 1.4) | 0.032 |
| 50–74.9 | 358 | Reference | 266 | Reference | 141 | Reference | |||
| ≥75 | 267 | −0.97 (−1.9, −0.1) | 0.037 | 519 | −0.14 (−1.0, 0.7) | 0.750 | 80 | 0.63 (−0.5, 1.7) | 0.262 |
| Trend2 | 819 | −0.39 (−0.9, 0.1) | 0.135 | 927 | 0.04 (−0.4, 0.5) | 0.871 | 1,351 | −0.21 (−0.6, 0.1) | 0.241 |
| Girls | |||||||||
| SBP | 389 | 443 | 638 | ||||||
| <25 | 6 | 0.35 (−5.5, 6.2) | 0.908 | 7 | −0.81 (−6.2, 4.6) | 0.768 | 95 | 0.11 (−1.6, 1.8) | 0.905 |
| 25–49.9 | 87 | −0.45 (−2.3, 1.4) | 0.639 | 60 | −0.86 (−3.0, 1.3) | 0.433 | 257 | 0.92 (−0.4, 2.2) | 0.157 |
| 0–49.9 | 93 | −0.40 (−2.2, 1.4) | 0.671 | 67 | −0.86 (−2.9, 1.2) | 0.418 | 352 | 0.71 (−0.5, 1.9) | 0.245 |
| 50–74.9 | 183 | Reference | 136 | Reference | 214 | Reference | |||
| ≥75 | 113 | −2.25 (−3.9, −0.5) | 0.010 | 240 | −0.08 (−1.6, 1.4) | 0.922 | 72 | 1.29 (−0.6, 3.2) | 0.175 |
| Trend2 | 389 | −0.95 (−1.9, 0.0) | 0.056 | 443 | 0.28 (−0.6, 1.2) | 0.533 | 638 | 0.07 (−0.6, 0.7) | 0.826 |
| DBP | 389 | 443 | 638 | ||||||
| <25 | 6 | −1.56 (−6.4, 3.2) | 0.522 | 7 | −2.58 (−7.0, 1.9) | 0.258 | 95 | 0.03 (−1.4, 1.5) | 0.971 |
| 25–49.9 | 87 | 0.08 (−01.5, 1.6) | 0.917 | 60 | −0.70 (−02.5, 1.1) | 0.444 | 257 | 0.71 (−0.4, 1.8) | 0.192 |
| 0–49.9 | 93 | −0.03 (−1.5, 1.5) | 0.972 | 67 | −0.89 (−2.6, 0.8) | 0.309 | 352 | 0.54 (−0.5, 1.5) | 0.296 |
| 50–74.9 | 183 | Reference | 136 | Reference | 214 | Reference | |||
| ≥75 | 113 | −0.72 (−2.1, 0.7) | 0.308 | 240 | 0.59 (−0.7, 1.8) | 0.355 | 72 | 1.06 (−0.5, 2.6) | 0.185 |
| Trend2 | 389 | −0.30 (−1.1, 0.5) | 0.459 | 443 | 0.71 (−0.0, 1.4) | 0.053 | 638 | 0.08 (−0.4, 0.6) | 0.756 |
| Boys | |||||||||
| SBP | 430 | 484 | 713 | ||||||
| <25 | 11 | 1.18 (−3.1, 5.5) | 0.591 | 6 | 4.47 (1.2, 10.1) | 0.120 | 121 | 1.66 (0.1, 3.2) | 0.037 |
| 25–49.9 | 90 | −0.46 (−2.3, 1.4) | 0.621 | 69 | −0.12 (−2.1, 1.9) | 0.903 | 314 | 0.22 (−1.0, 1.4) | 0.723 |
| 0–49.9 | 101 | −0.29 (−2.0, 1.5) | 0.748 | 75 | 0.23 (01.7, 2.2) | 0.817 | 435 | 0.62 (−0.5, 1.8) | 0.291 |
| 50–74.9 | 175 | Reference | 130 | Reference | 209 | Reference | |||
| ≥75 | 154 | −0.95 (−2.5, 0.6) | 0.218 | 279 | −0.87 (−2.3, 0.5) | 0.228 | 69 | 0.40 (−1.5, 2.3) | 0.679 |
| Trend2 | 430 | −0.41 (−1.2, 0.4) | 0.333 | 484 | −0.67 (−1.5, 0.1) | 0.102 | 713 | −0.45 (−1.0, 0.1) | 0.137 |
| DBP | 430 | 484 | 713 | ||||||
| <25 | 11 | −0.31 (−3.8, 3.1) | 0.859 | 6 | 5.53 (0.9, 10.2) | 0.020 | 121 | 1.29 (−0.0, 2.6) | 0.051 |
| 25–49.9 | 90 | −0.39 (−1.9, 1.1) | 0.603 | 69 | −0.32 (−2.0, 1.3) | 0.698 | 314 | 0.85 (−0.2, 1.9) | 0.100 |
| 0–49.9 | 101 | −0.38 (−1.8, 1.0) | 0.598 | 75 | 0.13 (−1.5, 1.7) | 0.879 | 435 | 0.97 (0.0, 1.9) | 0.046 |
| 50–74.9 | 175 | Reference | 130 | Reference | 209 | Reference | |||
| ≥75 | 154 | −1.15 (−2.4, 0.1) | 0.063 | 279 | −0.70 (−1.9, 0.5) | 0.240 | 69 | 0.17 (−1.4, 1.7) | 0.836 |
| Trend2 | 430 | −0.42 (−1.1, 0.3) | 0.219 | 484 | −0.55 (−1.2, 0.1) | 0.102 | 713 | −0.51 (−1.0, −0.0) | 0.043 |
| Interaction, girls vs. boys | |||||||||
| SBP | 819 | 0.776 | 927 | 0.200 | 1,351 | 0.081 | |||
| DBP | 819 | 0.697 | 927 | 0.012 | 1,351 | 0.426 | |||
Values are results of multiple linear regression. All models adjusted for maternal ethnicity, smoking during pregnancy, height at 5 y, weight at 5 y, and parity. s-25(OH)D concentration quartiles: cord blood: Q1, <31.1; Q2, 31.1–45; Q3, 45.5–60.7, Q4, ≥60.7 nmol/L; early pregnancy: Q1, <50.7; Q2, 50.7–65.5; Q3, 65.6–78.5; Q4, ≥78.5 nmol/L; late pregnancy: Q1, <60.3; Q2, 60.3–78.5; Q3, 78.6–95.8; Q4, ≥95.8 nmol/L. DBP, diastolic blood pressure; Q, quartile; SBP, systolic blood pressure; vs., versus; s-25(OH)D, serum 25-hydroxyvitamin D.
The test for trend is done for <25 nmol/L, 25–49.9 nmol/L, 50–74.9 nmol/L and ≥75 nmol/L.
TABLE 3.
Adjusted associations between 5-y SBP/DBP and quartiles of s-25(OH)D in pregnancy and cord blood1
| Association with s-25(OH)D concentration | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Early pregnancy | Late pregnancy | Cord blood | |||||||
| All participants | n | β (95% CI) | P value | n | β (95% CI) | P value | n | β (95% CI) | P value |
| SBP | 819 | 927 | 1,351 | ||||||
| Q1 | 203 | Reference | 229 | Reference | 321 | Reference | |||
| Q2 | 207 | 0.08 (−1.3, 1.5) | 0.913 | 232 | 0.34 (−0.9, 1.6) | 0.606 | 346 | −0.49 (−1.6, 0.6) | 0.365 |
| Q3 | 205 | −0.82 (−2.2, 0.6) | 0.248 | 234 | −0.05 (−1.3, 1.2) | 0.943 | 342 | −0.43 (−1.5, 0.6) | 0.432 |
| Q4 | 204 | −1.22 (−2.6, 0.2) | 0.090 | 232 | −0.30 (−1.6, 1.0) | 0.650 | 342 | −0.28 (−1.4, 0.8) | 0.604 |
| Trend | 819 | −0.46 (−0.9, −0.0) | 0.040 | 927 | −0.13(−0.5, 0.3) | 0.532 | 1351 | −0.07 (−0.4, 0.3) | 0.674 |
| DBP | 819 | 927 | 1,351 | ||||||
| Q1 | 203 | Reference | 229 | Reference | 321 | Reference | |||
| Q2 | 207 | 0.08 (−1.0, 1.2) | 0.888 | 232 | 0.06 (−1.0, 1.1) | 0.908 | 346 | −0.26 (−1.2, 0.6) | 0.564 |
| Q3 | 205 | −0.19 (−1.3, 0.9) | 0.741 | 234 | 0.41 (−0.7, 1.5) | 0.452 | 342 | −0.30 (−1.2, 0.6) | 0.517 |
| Q4 | 204 | −0.90 (−2.0, 0.2) | 0.120 | 232 | −0.24 (−1.3, 0.8) | 0.660 | 342 | −0.40 (−1.3, 0.5) | 0.376 |
| Trend | 819 | −0.30 (−0.7, 0.1) | 0.097 | 927 | −0.04 (−0.4, 0.3) | 0.822 | 1,351 | −0.12 (−0.4, 0.2) | 0.393 |
| Girls2 | |||||||||
| SBP | 389 | 443 | 638 | ||||||
| Q1 | 96 | Reference | 115 | Reference | 148 | Reference | |||
| Q2 | 110 | −0.21 (−2.2, 1.8) | 0.836 | 111 | 0.75 (−1.1, 2.6) | 0.428 | 152 | −0.44 (−2.0, 1.2) | 0.595 |
| Q3 | 95 | −0.69 (−2.8, 1.4) | 0.518 | 100 | 0.31 (−1.6, 2.2) | 0.751 | 166 | −0.22 (−1.8, 1.4) | 0.782 |
| Q4 | 88 | −1.70 (−3.9, 0.5) | 0.123 | 117 | 0.41 (−1.5, 2.3) | 0.664 | 172 | 0.04 (−1.5, 1.7) | 0.960 |
| Trend | 389 | −0.56 (−1.2, 0.1) | 0.104 | 443 | 0.08 (−0.5, 0.7) | 0.790 | 638 | 0.04 (−0.5, 0.5) | 0.863 |
| DBP | 389 | 443 | 638 | ||||||
| Q1 | 96 | Reference | 115 | Reference | 148 | Reference | |||
| Q2 | 110 | −0.22 (−1.9,1.4) | 0.796 | 111 | 0.42 (−1.1, 2.0) | 0.596 | 152 | −0.19 (−1.5, 1.2) | 0.778 |
| Q3 | 95 | −0.47 (−2.2, 1.2) | 0.584 | 100 | 1.35 (−0.3, 3.0) | 0.099 | 166 | −0.19 (−1.5, 1.1) | 0.773 |
| Q4 | 88 | −0.32 (−2.1, 1.4) | 0.723 | 117 | 0.64 (−0.9, 2.2) | 0.420 | 172 | 0.20 (−1.1, 1.5) | 0.765 |
| Trend | 389 | −0.12 (−0.7, 0.4) | 0.659 | 443 | 0.27 (−0.2, 0.8) | 0.285 | 638 | 0.07 (−0.3, 0.5) | 0.748 |
| Boys2 | |||||||||
| SBP | 430 | 484 | 713 | ||||||
| Q1 | 107 | Reference | 114 | Reference | 173 | Reference | |||
| Q2 | 97 | 0.50 (−1.5, 2.5) | 0.614 | 121 | −0.09 (−1.8, 1.7) | 0.922 | 194 | −0.57 (−2.0, 0.9) | 0.439 |
| Q3 | 110 | −0.86 (−2.8, 1.1) | 0.382 | 134 | −0.51 (−2.2, 1.2) | 0.563 | 176 | −0.56 (−2.0, 0.9) | 0.450 |
| Q4 | 116 | −0.79 (−2.7, 1.1) | 0.411 | 115 | −1.08 (−2.9, 0.7) | 0.239 | 170 | −0.51 (−2.0, 1.0) | 0.499 |
| Trend | 430 | −0.38 (−1.0, 0.2) | 0.217 | 484 | −0.37 (−0.9, 0.2) | 0.203 | 713 | −0.15 (−0.6, 0.3) | 0.529 |
| DBP | 430 | 484 | 713 | ||||||
| Q1 | 107 | Reference | 114 | Reference | 173 | Reference | |||
| Q2 | 97 | 0.44 (−1.1, 2.0) | 0.583 | 121 | −0.36 (−1.8, 1.1) | 0.626 | 194 | −0.34 (−1.5, 0.8) | 0.570 |
| Q3 | 110 | 0.11 (−1.4, 1.7) | 0.889 | 134 | −0.36 (−1.8, 1.1) | 0.615 | 176 | −0.38 (−1.6, 0.8) | 0.537 |
| Q4 | 116 | −1.21 (−2.7, 0.3) | 0.117 | 115 | −1.10 (−2.6, 0.4) | 0.146 | 170 | −1.00 (−2.2, 0.2) | 0.109 |
| Trend | 430 | −0.42 (−0.9, 0.1) | 0.086 | 484 | −0.33 (−0.8, 0.1) | 0.170 | 713 | −0.30 (−0.7, 0.1) | 0.123 |
Values are results of multiple linear regression. All models adjusted for maternal ethnicity, smoking during pregnancy, height at 5 y, weight at 5 y, and parity. s-25(OH)D quartiles: early pregnancy: Q1, <50.7; Q2, 50.7–65.5; Q3, 65.6–78.5; and Q4, ≥78.5 nmol/L; late pregnancy: Q1, <60.3; Q2, 60.3–78.5; Q3, 78.6–95.8; and Q4, ≥95.8 nmol/L. DBP, diastolic blood pressure; GA, gestational age; Q, quartile; SBP, systolic blood pressure; vs., versus; s-25(OH)D, serum 25-hydroxyvitamin D.
No interactions based on participant sex were found in any of the associations.
Applying Bonferroni correction for two outcomes, significant associations remained between continuous s-25(OH)D and SBP in the total cohort, and between s-25(OH)D >75 nmol/L and SBP in the total cohort and in girls (P < 0.025 for all). Tests for sex differences were not statistically significant (SBP; P = 0.445, DBP P = 0.857).
Late pregnancy and cord s-25(OH)D associations to blood pressure at 5 y
In the adjusted analysis, late pregnancy s-25(OH)D <25 nmol/L associated inversely with DBP in boys. Test for sex differences were not statistically significant (SBP; P = 0.163, DBP; P = 0.056). No other associations were found between late pregnancy s-25(OH)D and 5-y BP.
In cord blood, s-25(OH)D <50 nmol/L compared with reference (50-74.9 nmol/L) nmol/L associated to higher DBP (P < 0.05) in the total cohort. For boys, SBP were higher for cord s-25(OH)D <25 nmol/L compared with reference and DBP associated inversely with cord s-25(OH)D in test for trend and for <50 nmol/L compared with reference (P< 0.05 for all). However, the sex differences were not statistically significant (SBP; P = 0.210, DBP P = 0.067). No associations were found between cord s-25(OH)D and 5-y BP ≥90th percentile. No associations were observed in girls.
Two-stage analysis of overall association
The two-stage analysis combining early pregnancy, late pregnancy, and cord s-25(OH)D data showed an inverse association with both 5-y SBP and DBP for boys (P < 0.025), but not for girls or the total cohort (Table 5). Test for sex differences were statistically significant for DBP (P = 0.004), but not for SBP (P = 0.092).
TABLE 5.
Two-stage analysis, associations between 5-y SBP/DBP and vitamin D (early pregnancy, late pregnancy, and cord blood)1
| Association with 5-y SBP/DBP and vitamin D | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | β (95% CI) | P value | Girls | n | β (95% CI) | P value | Boys | n | β (95% CI) | P value | P interaction girls vs. boys | |
| SBP | 1351 | −0.02 (−0.04, 0.00) | 0.099 | 638 | 0.00 (−0.03, 0.04) | 0.861 | 713 | −0.04 (−0.07, −0.01) | 0.021 | 0.092 | ||
| DBP | 1351 | −0.02 (−0.04, 0.00) | 0.100 | 638 | 0.01 (−0.02, 0.05) | 0.393 | 713 | −0.04 (−0.07, −0.02) | 0.001 | 0.004 | ||
Values are results of 2-stage analysis (multiple regression) adjusted for maternal ethnicity, smoking during pregnancy, height at 5 y, weight at 5 y, and parity. DBP, diastolic blood pressure; SBP, systolic blood pressure; vs., versus; s-25(OH)D, serum 25-hydroxyvitamin D.
Other analyses
No consistent associations were detected between 5-y s-25(OH)D and 5-y SBP/DBP (Supplementary Tables 5–8). In sensitivity analyses, quartiles defined for girls and boys separately did not change our results for any of the associations studied (data not shown).
Likewise, our primary association remained unchanged when 1) excluding children born preterm, 2) excluding mothers with preeclampsia or gestational hypertension, or 3) adding maternal 1st trimester BP, or mean of 1st, 2nd, and 3rd trimester BP, as a covariate (data not shown).
Discussion
In our population-based cohort study, higher s-25(OH)D in early pregnancy was associated with lower child blood pressure at 5 y age. Split by routine cutoffs, s-25(OH)D >75 nmol/L associated with lower SBP and DBP. No associations with SBP or DBP >90th percentile or sex-specific associations were detected. For boys, inverse associations with blood pressure were found in the 2-stage model for overall pregnancy and cord vitamin D exposure.
Determinants of BP in childhood may include maternal factors already from early pregnancy. In a previous study, we identified positive associations between maternal first, second, and third trimester BP and offspring BP up to 5 y of age (38). In a large-scale genetic meta-analysis, BP in adults was linked to regions of active chromatin in fetal heart, muscle, kidney, and adrenal gland and lung tissues, suggesting a link between fetal development and later BP regulation (24).
Vitamin D plays an important role in the fetal development in many organs. In animal studies, vitamin D receptor gene deletion leads to activating of the renin-angiotensin system, hypertension, and target-organ damage (41) and similar effects are shown for 1α-hydroxylase gene knock out (42). Vitamin D deficiency during pregnancy increases the number of nephrons and glomeruli, but delay maturity of glomeruli in offspring (26, 27), and parental vitamin D depletion in rats leads to increased SBP and DBP with hypermethylation of the promotor region of the Panx1 and impaired endothelial relaxation (28). Conversely, calcitriol [1,25(OH)2D3] lowers renin expression through actions on the renin gene promoter (29).
No large RCTs have addressed the possible effect of vitamin D supplementation in pregnancy on offspring BP. However, in a small RCT (n = 52), BP was similar in the 12- to 16-mo-old offspring between groups with vitamin D supplementation or placebo in pregnancy (43).
Inverse associations between pregnancy or cord s-25(OH)D and offspring BP have been found in some (17–21), but not all (22, 23) observational studies. Of note, the large observational Dutch study by Miliku et al. (22), which did not find associations between s-25(OH)D in mid-gestation pregnancy and 6y BP in the offspring, adjusted for multivitamin supplementation and calcium intake in pregnancy, unlike our and other studies. As vitamin D supplementation is a major determinant for s-25(OH)D concentrations in pregnancy [Table 1 and previously shown (34)], adjusting for multivitamin supplementation may have masked a true association in the Dutch study.
We had the opportunity to explore 3 exposure times in early life and identified early pregnancy as the exposure time with most significant associations. In late pregnancy and cord blood, weaker associations were still present for boys and the 2-stage model for the combined exposure of early pregnancy, late pregnancy, and cord 25(OH)D were highly significant in boys, in keeping with the findings of males being more susceptible to developmental insults in early life with regard to cardiovascular health (44, 45).
Large-scale twin studies would be optimal to further address this sex difference.
We identified an inverse association between optimal vitamin D status in early pregnancy (s-25(OH)D ≥75 nmol/L) and SBP and DBP in the 5-y-old offspring, whereas vitamin D deficiency (<25 nmol/L) was not associated with SBP or DBP. This may suggest a beneficial role of optimal vitamin D status in early pregnancy on offspring BP. In former studies, only low vitamin D status in pregnancy (20), or the continuous full scale of s-25(OH)D (17–19, 21), showed associations with offspring BP.
Our mean s-25(OH)D concentrations were relatively high in early and late pregnancy compared with most other (46, 47), but not all, studies (48). A generally high vitamin D status in a cohort may lead to failure to detect an association between low vitamin D status and higher offspring BP, especially in late pregnancy where s-25(OH)D reach the highest concentrations, compatible with an increasing adherence to vitamin D supplementation recommendations during pregnancy in our cohort (34, 49). On the other hand, the failure to detect associations between optimal vitamin D status and lower BP in late pregnancy or cord suggests a time window of a protective effect of optimal vitamin D status on offspring BP confined to early pregnancy.
The lack of association between 5-y s-25(OH)D and BP is in keeping with null effect of vitamin D supplementation in childhood on BP in RCTs (11). However, our cohort was not suitable for studying associations of very low s-25(OH)D concentrations, nor at 5 y.
We noted that the mean SBP/DBP of 101/64 in our cohort was high compared with the consensus guideline BP pediatric reference (50), but only slightly higher than the mean SBP/DBP of 100/63 at 3 y of age (21). These high values can partly be ascribed to the oscillometric method used in OCC, partly to a mean 5-y height in OCC comparable with the 75th percentile height in the consensus reference population (data not shown). These differences were not believed to bias the associations studied.
Strengths and limitations
Strengths of this study included the use of a large population-based birth cohort, the longitudinal s-25(OH)D sampling, the use of s-25(OH)D measured by gold standard method instead of questionnaire data on vitamin D supplementation, and child examination performed by trained staff blinded for s-25(OH)D. Limitations of the study included the observational nature of our study with the use of self-reported data in covariates and the potential for chance findings and residual confounding. Moreover, eligible pregnant women who did not participate in the OCC at all were more likely to have higher parity, to smoke during pregnancy, be of non-Western ethnicity, and have children with darker skin in a previously reported selection bias analysis (35).
In conclusion, early pregnancy s-25(OH)D was inversely associated to measures of SBP and DBP at 5 y with a novel identified inverse association between optimal vitamin D status [s-25(OH)D >75 nmol/L] and BP at 5 y. Mixed effect models for pregnancy and cord s-25(OH)D identified an inverse association in the male offspring only.
Our findings may encourage women to obtain optimal vitamin D status already before or within early pregnancy. Although the associations led to only small differences in SBP and DBP and no associations to BP >90th percentile, the results may have clinical importance given the strong evidence for higher BP tracking from childhood into adulthood (9, 51) with association to clinical hypertension (9, 52, 53) and metabolic syndrome (53).
In the policy making of public health recommendations on vitamin D supplementation in pregnancy, the potential small beneficial effect on offspring BP must, however, be balanced against the risk of vitamin D toxicity. Longer follow-ups into adolescence and adulthood and high-evidence data from well-designed RCTs should address the question of vitamin D supplementation earliest possible in pregnancy with respect to offspring BP along with other outcomes.
Supplementary Material
ACKNOWLEDGEMENTS
We acknowledge the research staff at the Hans Christian Andersen children's Hospital for their careful examinations of the study participants. We are grateful to the OCC participants for their use of time and engagement in the cohort.
The authors’ responsibilities were as follows – JNP, CD, HTC: designed the study; JNP, AB, MSA: collected the data; JNP, SM, CD: analyzed data; JNP, CD, LBA, SM, HTC: wrote the paper; HTC: had primary responsibility for final content; and all authors: read and approved the final manuscript. The authors report no conflicts of interest.
Notes
Supported by the AP Møller foundation of medical research (jr.nr. 18-L0294) and Beckett-Fonden (jr.nr. 18-2-1818). Both applications done by JNP. These funds had no influence on the design, implementation, analysis, or interpretation of the study.
Supplementary Figures 1 and 2 and Supplementary Tables 1–8 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/jn/.
Abbreviations used: BP, blood pressure; DBP, diastolic blood pressure; lowess, locally weighted scatterplot smoothing; OCC, Odense Child Cohort; RCT, randomized controlled trials; SBP, systolic blood pressure; s-25(OH)D, S-25-hydroxyvitamin D.
Contributor Information
Josefine N Pedersen, Department of Clinical Research, Faculty of Health Sciences, University of Southern Denmark, Odense, Denmark; Hans Christian Andersen Children's Hospital, Odense University Hospital, Odense, Denmark.
Christine Dalgård, Department of Clinical Research, Faculty of Health Sciences, University of Southern Denmark, Odense, Denmark; Clinical Pharmacology, Pharmacy and Environmental Medicine, Dept of Public Health, University of Southern Denmark, Odense, Denmark.
Sören Möller, Department of Clinical Research, Faculty of Health Sciences, University of Southern Denmark, Odense, Denmark; Open Patient data Explorative Network, Odense University Hospital, Odense, Denmark.
Louise B Andersen, General Practice, Capital Region, Denmark; Department of Obstetrics and Gynecology, Odense University Hospital, Odense, Denmark.
Anna Birukov, Department of Molecular Epidemiology, German Institute of Human Nutrition Potsdam-Rehbrücke, Nuthetal, Germany.
Marianne Skovsager Andersen, Open Patient data Explorative Network, Odense University Hospital, Odense, Denmark; Department of Endocrinology, Odense University Hospital, University of Southern Denmark, Odense, Denmark.
Henrik T Christesen, Department of Clinical Research, Faculty of Health Sciences, University of Southern Denmark, Odense, Denmark; Hans Christian Andersen Children's Hospital, Odense University Hospital, Odense, Denmark; Odense Child Cohort, Odense University Hospital, Odense, Denmark.
Data Availability
Data described in the manuscript, code book, and analytic code will not be made available as they contain personal information on the participants.
References
- 1. Magnussen CG, Smith KJ. Pediatric blood pressure and adult preclinical markers of cardiovascular disease. Clin Med Insights Blood Disord. 2016;9:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Beveridge LA, Struthers AD, Khan F, Jorde R, Scragg R, Macdonald HMet al. Effect of vitamin D supplementation on blood pressure: a systematic review and meta-analysis incorporating individual patient data. JAMA Intern Med. 2015;175(5):745–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang D, Cheng C, Wang Y, Sun H, Yu S, Xue Yet al. Effect of vitamin D on blood pressure and hypertension in the general population: an update meta-analysis of cohort studies and randomized controlled trials. Prev Chronic Dis. 2020;17:E03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wu L, Sun D. Effects of calcium plus vitamin D supplementation on blood pressure: a systematic review and meta-analysis of randomized controlled trials. J Hum Hypertens. 2017;31(9):547–54. [DOI] [PubMed] [Google Scholar]
- 5. Kunutsor SK, Burgess S, Munroe PB, Khan H. Vitamin D and high blood pressure: causal association or epiphenomenon?. Eur J Epidemiol. 2014;29(1):1–14. [DOI] [PubMed] [Google Scholar]
- 6. Kunutsor SK, Apekey TA, Steur M. Vitamin D and risk of future hypertension: meta-analysis of 283,537 participants. Eur J Epidemiol. 2013;28(3):205–21. [DOI] [PubMed] [Google Scholar]
- 7. Vimaleswaran KS, Cavadino A, Berry DJ, Jorde R, Dieffenbach AK, Lu Cet al. Association of vitamin D status with arterial blood pressure and hypertension risk: a mendelian randomisation study. Lancet Diabetes Endocrinol. 2014;2(9):719–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen S, Sun Y, Agrawal DK. Vitamin D deficiency and essential hypertension. J Am Soc Hypertens. 2015;9(11):885–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: a systematic review and meta-regression analysis. Circulation. 2008;117(25):3171–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kelly RK, Thomson R, Smith KJ, Dwyer T, Venn A, Magnussen CG. Factors affecting tracking of blood pressure from childhood to adulthood: the childhood determinants of adult health study. J. Pediatr. 2015;167(6):1422–8.e2.e2. [DOI] [PubMed] [Google Scholar]
- 11. Abboud M. Vitamin D supplementation and blood pressure in children and adolescents: a systematic review and meta-analysis. Nutrients. 2020;12(4):1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pacifico L, Anania C, Osborn JF, Ferraro F, Bonci E, Olivero Eet al. Low 25(OH)D3 levels are associated with total adiposity, metabolic syndrome, and hypertension in Caucasian children and adolescents. Eur J Endocrinol. 2011;165(4):603–11. [DOI] [PubMed] [Google Scholar]
- 13. Ganji V, Zhang X, Shaikh N, Tangpricha V. Serum 25-hydroxyvitamin D concentrations are associated with prevalence of metabolic syndrome and various cardiometabolic risk factors in US children and adolescents based on assay-adjusted serum 25-hydroxyvitamin D data from NHANES 2001-2006. Am J Clin Nutr. 2011;94(1):225–33. [DOI] [PubMed] [Google Scholar]
- 14. Tomaino K, Romero KM, Robinson CL, Baumann LM, Hansel NN, Pollard SLet al. Association between serum 25-hydroxy vitamin D levels and blood pressure among adolescents in two resource-limited settings in Peru. Am J Hypertens. 2015;28(8):1017–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Petersen RA, Dalskov SM, Sørensen LB, Hjorth MF, Andersen R, Tetens Iet al. Vitamin D status is associated with cardiometabolic markers in 8-11-year-old children, independently of body fat and physical activity. Br J Nutr. 2015;114(10):1647–55. [DOI] [PubMed] [Google Scholar]
- 16. Dolinsky DH, Armstrong S, Mangarelli C, Kemper AR. The association between vitamin D and cardiometabolic risk factors in children: a systematic review. Clin Pediatr (Phila). 2013;52(3):210–23. [DOI] [PubMed] [Google Scholar]
- 17. Rytter D, Bech BH, Halldorsson TI, Henriksen TB, Grandström C, Cohen Aet al. Maternal vitamin D status at week 30 of gestation and offspring cardio-metabolic health at 20 years: a prospective cohort study over two decades. PLoS One. 2016;11(10):e0164758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Williams DM, Fraser A, Fraser WD, Hyppönen E, Davey Smith G, Deanfield Jet al. Associations of maternal 25-hydroxyvitamin D in pregnancy with offspring cardiovascular risk factors in childhood and adolescence: findings from the Avon Longitudinal Study of Parents and Children. Heart. 2013;99(24):1849–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sauder KA, Stamatoiu AV, Leshchinskaya E, Ringham BM, Glueck DH, Dabelea D. Cord blood vitamin D levels and early childhood blood pressure: the Healthy Start Study. J Am Heart Assoc. 2019;8(9):e011485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang G, Liu X, Bartell TR, Pearson C, Cheng TL, Wang X. Vitamin D trajectories from birth to early childhood and elevated systolic blood pressure during childhood and adolescence. Hypertension. 2019;74(2):421–30.. Hypertensionaha11913120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Larsen SD, Dalgård C, Christensen ME, Lykkedegn S, Andersen LB, Andersen Met al. Blood pressure in 3-year-old girls associates inversely with umbilical cord serum 25-hydroxyvitamin D: an Odense Child Cohort study. Endocr Connect. 2018;7(12):1236–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Miliku K, Felix JF, Voortman T, Tiemeier H, Eyles DW, Burne THet al. Associations of maternal and fetal vitamin D status with childhood body composition and cardiovascular risk factors. Matern Child Nutr. 2019;15(2):e12672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Krishnaveni GV, Veena SR, Winder NR, Hill JC, Noonan K, Boucher BJet al. Maternal vitamin D status during pregnancy and body composition and cardiovascular risk markers in Indian children: the Mysore Parthenon Study. Am J Clin Nutr. 2011;93(3):628–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Surendran P, Feofanova EV, Lahrouchi N, Ntalla I, Karthikeyan S, Cook Jet al. Discovery of rare variants associated with blood pressure regulation through meta-analysis of 1.3 million individuals. Nat Genet. 2020;52(12):1314–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ritz E, Amann K, Koleganova N, Benz K. Prenatal programming—effects on blood pressure and renal function. Nat Rev Nephrol. 2011;7(3):137–44. [DOI] [PubMed] [Google Scholar]
- 26. Nascimento FA, Ceciliano TC, Aguila MB, Mandarim-de-Lacerda CA. Maternal vitamin D deficiency delays glomerular maturity in F1 and F2 offspring. PLoS One. 2012;7(8):e41740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Maka N, Makrakis J, Parkington HC, Tare M, Morley R, Black MJ. Vitamin D deficiency during pregnancy and lactation stimulates nephrogenesis in rat offspring. Pediatr Nephrol. 2008;23(1):55–61. [DOI] [PubMed] [Google Scholar]
- 28. Meems LM, Mahmud H, Buikema H, Tost J, Michel S, Takens Jet al. Parental vitamin D deficiency during pregnancy is associated with increased blood pressure in offspring via Panx1 hypermethylation. Am J Physiol Heart Circ Physiol. 2016;311(6):H1459–69. [DOI] [PubMed] [Google Scholar]
- 29. Yuan W, Pan W, Kong J, Zheng W, Szeto FL, Wong KEet al. 1,25-dihydroxyvitamin D3 suppresses renin gene transcription by blocking the activity of the cyclic AMP response element in the renin gene promoter. J. Biol Chem. 2007;282(41):29821–30. [DOI] [PubMed] [Google Scholar]
- 30. Moritz KM, Dodic M, Wintour EM. Kidney development and the fetal programming of adult disease. Bioessays. 2003;25(3):212–20. [DOI] [PubMed] [Google Scholar]
- 31. Saraf R, Morton SM, Camargo CA Jr., Grant CC. Global summary of maternal and newborn vitamin D status—a systematic review. Matern Child Nutr. 2016;12(4):647–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Holick MF. The vitamin D deficiency pandemic: approaches for diagnosis, treatment and prevention. Rev Endocr Metab Disord. 2017;18(2):153–65. [DOI] [PubMed] [Google Scholar]
- 33. Andersen LB, Abrahamsen B, Dalgard C, Kyhl HB, Beck-Nielsen SS, Frost-Nielsen Met al. Parity and tanned white skin as novel predictors of vitamin D status in early pregnancy: a population-based cohort study. Clin Endocrinol (Oxf). 2013;79(3):333–41. [DOI] [PubMed] [Google Scholar]
- 34. Lykkedegn S, Beck-Nielsen SS, Sorensen GL, Andersen LB, Fruekilde PBN, Nielsen Jet al. Vitamin D supplementation, cord 25-hydroxyvitamin D and birth weight: Findings from the Odense Child Cohort. Clin Nutr. 2017;36(6):1621–7. [DOI] [PubMed] [Google Scholar]
- 35. Kyhl HB, Jensen TK, Barington T, Buhl S, Norberg LA, Jørgensen JSet al. The Odense Child Cohort: aims, design, and cohort profile. Paediatr Perinat Epidemiol. 2015;29(3):250–8. [DOI] [PubMed] [Google Scholar]
- 36. Sempos CT, Heijboer AC, Bikle DD, Bollerslev J, Bouillon R, Brannon PMet al. Vitamin D assays and the definition of hypovitaminosis D: results from the First International Conference on Controversies in Vitamin D. Br J Clin Pharmacol. 2018;84(10):2194–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Eşer I, Khorshid L, Güneş UY, Demir Y. The effect of different body positions on blood pressure. J Clin Nurs. 2007;16(1):137–40. [DOI] [PubMed] [Google Scholar]
- 38. Birukov A, Herse F, Nielsen JH, Kyhl HB, Golic M, Kräker Ket al. Blood pressure and angiogenic markers in pregnancy: contributors to pregnancy-induced hypertension and offspring cardiovascular risk. Hypertension. 2020;76(3):901–9. [DOI] [PubMed] [Google Scholar]
- 39. Fitzpatrick TB. The validity and practicality of sun-reactive skin types I through VI. Arch Dermatol. 1988;124(6):869–71. [DOI] [PubMed] [Google Scholar]
- 40. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–9. [DOI] [PubMed] [Google Scholar]
- 41. Li YC, Kong J, Wei M, Chen ZF, Liu SQ, Cao LP. 1,25-Dihydroxyvitamin D(3) is a negative endocrine regulator of the renin-angiotensin system. J Clin Invest. 2002;110(2):229–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhou C, Lu F, Cao K, Xu D, Goltzman D, Miao D. Calcium-independent and 1,25(OH)2D3-dependent regulation of the renin-angiotensin system in 1alpha-hydroxylase knockout mice. Kidney Int. 2008;74(2):170–9. [DOI] [PubMed] [Google Scholar]
- 43. Sahoo SK, Katam KK, Das V, Agarwal A, Bhatia V. Maternal vitamin D supplementation in pregnancy and offspring outcomes: a double-blind randomized placebo-controlled trial. J Bone Miner Metab. 2017;35(4):464–71. [DOI] [PubMed] [Google Scholar]
- 44. Ojeda NB, Intapad S, Alexander BT. Sex differences in the developmental programming of hypertension. Acta Physiol (Oxf). 2014;210(2):307–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Dasinger JH, Alexander BT. Gender differences in developmental programming of cardiovascular diseases. Clin Sci (Colch). 2016;130(5):337–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Johnson DD, Wagner CL, Hulsey TC, McNeil RB, Ebeling M, Hollis BW. Vitamin D deficiency and insufficiency is common during pregnancy. Am J Perinatol. 2011;28(01):007–12. [DOI] [PubMed] [Google Scholar]
- 47. Moon RJ, Crozier SR, Dennison EM, Davies JH, Robinson SM, Inskip HMet al. Tracking of 25-hydroxyvitamin D status during pregnancy: the importance of vitamin D supplementation. Am J Clin Nutr. 2015;102(5):1081–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Magnus MC, Stene LC, Håberg SE, Nafstad P, Stigum H, London SJet al. Prospective study of maternal mid-pregnancy 25-hydroxyvitamin D level and early childhood respiratory disorders. Paediatr Perinat Epidemiol. 2013;27(6):532–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Andersen LB, Abrahamsen B, Dalgård C, Kyhl HB, Beck-Nielsen SS, Frost-Nielsen Met al. Parity and tanned white skin as novel predictors of vitamin D status in early pregnancy: a population-based cohort study. Clin Endocrinol (Oxf). 2013;79(3):333–41. [DOI] [PubMed] [Google Scholar]
- 50. Lurbe E, Agabiti-Rosei E, Cruickshank JK, Dominiczak A, Erdine S, Hirth Aet al. 2016 European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J Hypertens. 2016;34(10):1887–920. [DOI] [PubMed] [Google Scholar]
- 51. Theodore RF, Broadbent J, Nagin D, Ambler A, Hogan S, Ramrakha Set al. Childhood to early-midlife systolic blood pressure trajectories: early-life predictors, effect modifiers, and adult cardiovascular outcomes. Hypertension. 2015;66(6):1108–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Juhola J, Oikonen M, Magnussen CG, Mikkilä V, Siitonen N, Jokinen Eet al. Childhood physical, environmental, and genetic predictors of adult hypertension: the cardiovascular risk in young Finns study. Circulation. 2012;126(4):402–9. [DOI] [PubMed] [Google Scholar]
- 53. Sun SS, Grave GD, Siervogel RM, Pickoff AA, Arslanian SS, Daniels SR. Systolic blood pressure in childhood predicts hypertension and metabolic syndrome later in life. Pediatrics. 2007;119(2):237–46. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data described in the manuscript, code book, and analytic code will not be made available as they contain personal information on the participants.