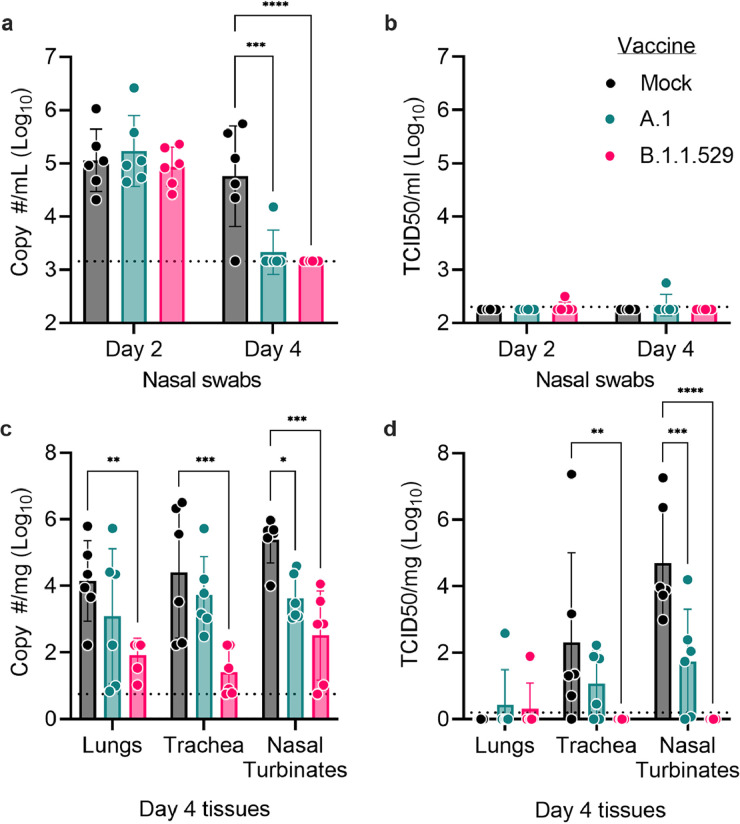
Figure 3.
Vaccine protective efficacy against B.1.1.529 infection of Syrian hamsters. Following intranasal infection of vaccinated hamsters with 1000 tissue culture 50% infectious doses (TCID50), nasal swabs were collected on days 2 and 4 for evaluation of (a) viral RNA load by reverse transcription quantitative polymerase chain reaction (RT-qPCR) or for (b) infectious virus by TCID50 assay. On day 4, animals were sacrificed and lungs, trachea, as well as nasal turbinates harvested for quantification of (c) viral RNA load by RT-qPCR or (d) infectious virus by TCID50 assay. Indicated statistical comparisons performed using two-way ANOVA with Dunnett's multiple comparisons test. *p<0.05. Comparisons without indicated p-values were non-significant (p>0.05). N = 6 per group.