Abstract
Mother-to-child transmission of Helicobacter pylori (H. pylori) is the primary source of intrafamilial spread in early childhood in regions of high H. pylori prevalence. However, early-in-life H. pylori colonization and associated protective or risk factors have not been fully evaluated in lower prevalence regions, such as the United States.
Therefore, from a well-characterized prospective US cohort, we selected women who provided fecal samples during pregnancy and had paired fecal samples from their babies up to 24 months postpartum. We evaluated maternal and baby factors associated with likelihood of H. pylori colonization in the babies. Fecal antigen testing was used to determine H. pylori status. We also evaluated the association between maternal breastmilk cytokines and H. pylori colonization in breastfed babies.
Among included mother-baby pairs (n=66), H. pylori prevalence was 31.8% in mothers and 19.7% in their babies. Dominant breastfeeding (adjusted odds ratio [aOR] 0.17, 95% CI 0.03–0.98) and maternal IBD (aOR 0.05, 95% CI 0.01–0.27) were associated with significantly lower likelihood of H. pylori colonization among babies; no other clinical factors were associated with H. pylori colonization in the babies. Matrix metalloproteinase-10 (MMP-10) and tumor necrosis factor-related activation-induced cytokine (TRANCE) expression were significantly higher in breastmilk of mothers with H. pylori positive vs negative babies.
Consistent with data from high H. pylori prevalence regions, our findings suggest dominant breastfeeding may protect against early H. pylori colonization. Downregulation of pro-inflammatory cytokines such as MMP-10 may be relevant in mediating this protection among breastfed babies, but more data are needed.
Keywords: cytokines, breastfeeding, antibiotics, immunomodulation, Helicobacter pylori, infectious diseases
INTRODUCTION
Helicobacter pylori (H. pylori) is the most common chronic bacterial infection and is estimated to colonize over 50% of the population worldwide.1 H. pylori is well-known for its causal association with gastric cancer and gastroduodenal pathology, namely peptic ulcer disease. Albeit less appreciated, H. pylori also has immunomodulatory effects, and colonization early-in-life may protect against allergic, atopic and immune-mediated diseases, such as asthma and inflammatory bowel disease.2–4
In areas where H. pylori infection is endemic, such as Africa and Asia-Pacific countries, acquisition of infection typically occurs in childhood prior to 5–10 years of age.5–7 However, most studies have not investigated H. pylori acquisition very early in life, including the first two years of life, which is a key time for immune development. Prior studies have also relied on H. pylori serology, which does not necessarily indicate active H. pylori colonization in the infant, since H. pylori antibodies can be transmitted from mother to baby via cord transfer or breastmilk.8,9 Furthermore, no studies to our knowledge have investigated H. pylori acquisition in babies younger than 2 years-old in populations where H. pylori is not considered endemic, nor evaluated risk determinants in these populations. Maternal H. pylori status is a strong predictor of H. pylori status in offspring.10 Notably, several studies from high H. pylori prevalence countries have demonstrated that maternal breastfeeding, a practice that necessitates close physical contact of mothers and babies, is actually associated with lower likelihood of H. pylori positivity in babies. However, the few studies from lower prevalence countries that exist have not confirmed this.11,12
The primary objective of this study was to identify factors associated with early-in-life H. pylori colonization among a US cohort. We hypothesized that breastfeeding would be associated with lower likelihood of H. pylori colonization. If confirmed, our secondary objective was to conduct an exploratory analysis to evaluate whether breastmilk cytokine patterns are associated with likelihood of H. pylori colonization among breastfed babies.
METHODS
Study subjects
The base cohort for this study was the MECONIUM (Exploring MEChanisms Of disease traNsmission In Utero through the Microbiome) cohort, which is a well-characterized longitudinal cohort that contains longitudinal stool samples for pregnant mothers and their babies postpartum, along with comprehensive clinical data obtained at each collection time point; a detailed description of this cohort is provided elsewhere.13 Briefly, pregnant women with or without inflammatory bowel disease (IBD) were prospectively enrolled between March 2015 and August 2018. Clinical data and biospecimens were collected from pregnant women and their offspring postpartum at prespecified time points according to standardized protocol. For this study, we selected 66 mother-baby pairs who had fecal specimens and complete clinical data available. We tested maternal stool samples obtained during pregnancy and baby stool samples at 1 month, 3 months, 12 months, and 24 months postpartum for active H. pylori infection (see below). The exclusion criteria for the complete MECONIUM cohort were: no informed consent; maternal preexisting conditions of HIV/AIDS or autoimmune diseases, active infection (other than H. pylori) during pregnancy, or fetal chromosomal or structural abnormalities. For this study, mothers with reported proton pump inhibitor (PPI) use were excluded because this can affect H. pylori stool antigen test sensitivity.
Clinical Data Collection
The following demographic and obstetric data were collected for mothers: age at conception, race/ethnicity, smoking status, IBD status, IBD disease activity, and medications (including IBD-related medications, antibiotics, and probiotics). The following data were collected for babies: gestational age at delivery (prematurity defined as delivery <37 weeks of gestation), sex, delivery mode (vaginal delivery vs. Cesarean-section), birth weight (low birth weight defined as <2500 grams), and admission to the intensive care unit. Infants’ exposure to antibiotics after birth was also captured at the time of each stool sample collection. Data on infant feeding practices were determined from mothers’ self-report according to a standardized questionnaire administered at each collection timepoint and were categorized as: 1/ dominant breastfeeding if mothers reported breastfeeding for at least 75% of infant feeds; 2/ dominant formula feeding if mothers reported formula feeding for at least 75% of infant feeds; or 3/ mixed feeding if outside of these predetermined cut-offs.
Stool sample collection
Maternal stool sample collection was conducted at home by the mothers and shipped on ice to the study institution using prepaid labeled boxes; collections occurred at each trimester of pregnancy. Babies’ stool samples were obtained directly from diapers collected at 1 month, 3 months, 12 months, and 24 months after birth. Stool samples were received in 25 ml vials (adult) and diapers (infant) and stored at −80C until the time of H. pylori testing.
H. pylori ELISA
Active H. pylori gastric colonization was determined via ELISA-based testing for fecal H. pylori antigens (Epitope Diagnostics Inc., San Diego, CA, USA). Briefly, stool samples were weighed and diluted to 1:24 with assay buffer followed by extraction using intensive shaking via TissueLyser II (Qiagen, USA). After centrifugation for 5 min at 10,000 rpm at room temperature, 100 ul of samples (in duplicate) were loaded in microtiter plates coated with highly purified monoclonal anti-H. pylori antibodies and incubated for 60 minutes at room temperature. The wells were washed with wash buffer 4–5 times to remove non-binding material, and then incubated again with horseradish peroxidase-conjugated (HRP) monoclonal anti-H. pylori antibodies for 30 minutes at room temperature, followed by washing and incubation with HRP substrate (tetramethylbenzidine with hydrogen peroxidase). After 10–15 minutes of incubation in the dark, the reaction was stopped with 0.5M sulfuric acid. Optical density at 450/620 nm was determined by Synergy H1 hybrid multi-mode microplate reader (BioTek Instruments, US). As recommended by manufacturer, the positive and negative cut-offs were estimated by the following formula: Positive = 1.1 × (mean extinction of negative control + 0.10); negative = 0.9 × (mean extinction of negative control + 0.10).
Proteomics multiplex assay of breastmilk
For the exploratory analysis, we tested the hypothesis that breastmilk cytokine expression pattern is associated with likelihood of H. pylori colonization in breastfed babies. Among mothers who reported dominant (n=28) or mixed (n=13) breastfeeding, we evaluated maternal breastmilk cytokine expression profiles in samples obtained at approximately 1 month postpartum used Proseek® multiplex assay (Olink Bioscience, Uppsala, Sweden).14 The Proseek® multiplex assay uses a proximity extension assay technology with oligonucleotide-labeled antibody probe pairs that bind to their respective targets.14,15 Normalized protein expression values of 92 inflammation-related protein biomarkers were generated on a Log2 scale to normalize data to minimize intra-assay and inter-assay variability (please refer to “additional file 2” in the publication by Santaella et al.).16,17
Statistical analysis
We calculated maternal and infant H. pylori prevalence as the number of mothers or babies with positive H. pylori tests divided by the total number of mothers or babies tested, respectively. The student t-test or Wilcoxon rank-sum test and χ2 or Fisher’s Exact test were used to compare the values of continuous and categorical variables, respectively, between H. pylori positive vs negative study participants. We used multivariable regression modeling to estimate the association between clinical factors and H. pylori colonization status in babies. The models were adjusted for maternal age, smoking, maternal IBD status, maternal antibiotic usage, maternal H. pylori status, baby sex, delivery mode, type of feeding (dominant breastfeeding, formula feeding or mixed), and infant antibiotic usage.
For the breastmilk cytokine expression analysis, we selected the top differential features associated with H. pylori infection in mothers or infants based on “variable’s importance” (VIP) scores from a partial least squares-discriminant analysis (PLS-DA) model, which is a supervised clustering analysis method. A threshold VIP score >1.5 was used for defining statistically significant discriminative features.18,19 We conducted a multivariable logistic regression model adjusted for clinical variables including maternal IBD status, delivery mode, feeding type and infant antibiotic usage to estimate the association between cytokines identified as significant in the PLS-DA supervised model and H. pylori colonization status in breastfed babies. R version 4.0.3 was used for statistical analyses.
Ethics Statement
This study was approved by Institutional Review Board of the Icahn School of Medicine at Mount Sinai.
RESULTS
Study population
A total of 66 mother-baby pairs from the MECONIUM cohort met full inclusion criteria and were included in this study (Table 1). We tested 66 maternal stool samples at the 3rd trimester of their pregnancy and 153 total baby stool samples, which were available for each of the 66 babies at the distinct time points illustrated in Figure 1. Based on fecal antigen testing, 21 of 66 (31.8%) mothers and 13 of 66 (19.7%) babies tested positive for H. pylori. The descriptive characteristics of the 66 mother-baby pairs according to H. pylori status are provided in Table 1. Mothers who tested positive for H. pylori were slightly younger (34.6±4.0 vs 35.8 ±3.9 years old), less often non-Hispanic white, more often reported current/former smoking (14.3% vs 11.1%), and less often reported antibiotic usage (35.6% vs 19.0%), but these differences were not statistically significant, possibly due to small cohort size. IBD was more common in mothers with vs without H. pylori.
Table 1.
Baseline characteristics of mothers and their babies (n=66 biological mother-baby pairs) Univariate analysis
| Maternal Variables | H. pylori status† | ||
|---|---|---|---|
| Positive (n=21) | Negative (n=45) | p-value | |
| Age at enrollment (SD), years | 34.6 (4.0) | 35.8 (3.9) | 0.30 |
| Race | 0.11 | ||
| White | 17 (81.0%) | 41 (91.1%) | |
| Asian | 0 (0%) | 2 (4.4%) | |
| Black | 2 (9.5%) | 2 (4.4%) | |
| Other | 2 (9.5%) | 0 (0%) | |
| Hispanic ethnicity (Yes) | 2 (9.5%) | 4 (8.9%) | 1 |
| Smoking (current or former) (Yes) | 3 (14.3%) | 5 (11.1%) | 0.75 |
| Antibiotic usage (Yes) | 4 (19.0%) | 16 (35.6%) | 0.43 |
| IBD (Yes) | 4 (19.0%) | 34 (75.6%) | <0.001 |
| -IBD type (UC vs CD) | 1 (4.8%) vs 3 (14.3%) | 13 (28.9%) vs 14 (31.1%) | 0.61 |
| Babies’ Variables | H. pylori status† | ||
| Positive (n=13) | Negative (n=53) | p-value | |
| Mother H. pylori status (positive) | 6 (46.2%) | 15 (28.3%) | 0.32 |
| Mother IBD status (Yes) | 6 (46.2%) | 32 (60.4%) | 0.37 |
| Sex (Female) | 5 (38.5%) | 25 (47.2%) | 0.55 |
| Race‡ | 0.015 | ||
| White | 10 (76.9%) | 48 (90.6%) | |
| Asian | 1 (7.7%) | 1 (1.9%) | |
| Black | 0 (0%) | 4 (7.5%) | |
| Other | 2 (15.4%) | 0 (0%) | |
| Hispanic ethnicity (Yes)‡ | 0 (0%) | 6 (11.3%) | 0.59 |
| Gestational age (SD), months | 39.1 (1.2) | 39.3 (1.1) | 0.47 |
| C-section | 2 (15.4%) | 18 (34.0%) | 0.31 |
| Birth weight (SD), kg | 3.4 (0.4) | 3.3 (0.4) | 0.53 |
| Head circumference (SD), cm | 34.5 (2.3) | 35.0 (2.6) | 0.59 |
| Length SD, cm | 50.2 (2.5) | 49.7 (5.3) | 0.61 |
| Antibiotic exposure | 5 (38.5%) | 27 (50.9%) | 0.54 |
| Dominant breastfeeding | 2 (15.4%) | 26 (49.1%) | 0.032 |
| Day care attendance§ | 2 (15.3%) | 13 (24.5%) | 0.72 |
| Variables | Odds ratio | p-value |
|---|---|---|
| Maternal IBD* | 0.05 (0.01–0.27) | <0.001 |
| Dominant breastfeeding ** | 0.17 (0.029–0.98) | 0.049 |
Note: Subjects were categorized as H. pylori positive if any of the samples tested positive for H. pylori based on fecal antigen testing, and negative if all of the tested samples were negative.
Race and ethnicity information were based on mothers
At least 2 or more days per week attendance in daycare qualified as “daycare attendance”
p-values from multivariable logistic regression to test the association between maternal H. pylori status and the maternal IBD status after adjusting for maternal antibiotic usage, age, and smoking status.
p-values from multivariable logistic regression to test the association between infant H. pylori status and the dominant breastfeeding after adjusting for maternal H. pylori status, maternal IBD status, delivery mode, and antibiotic usage.
Abbreviations: SD, standard deviation; IBD, Inflammatory bowel disease; CD, Crohn’s disease; UC, Ulcerative colitis.
Figure 1. Mapping of H. pylori status within mother-baby dyads.
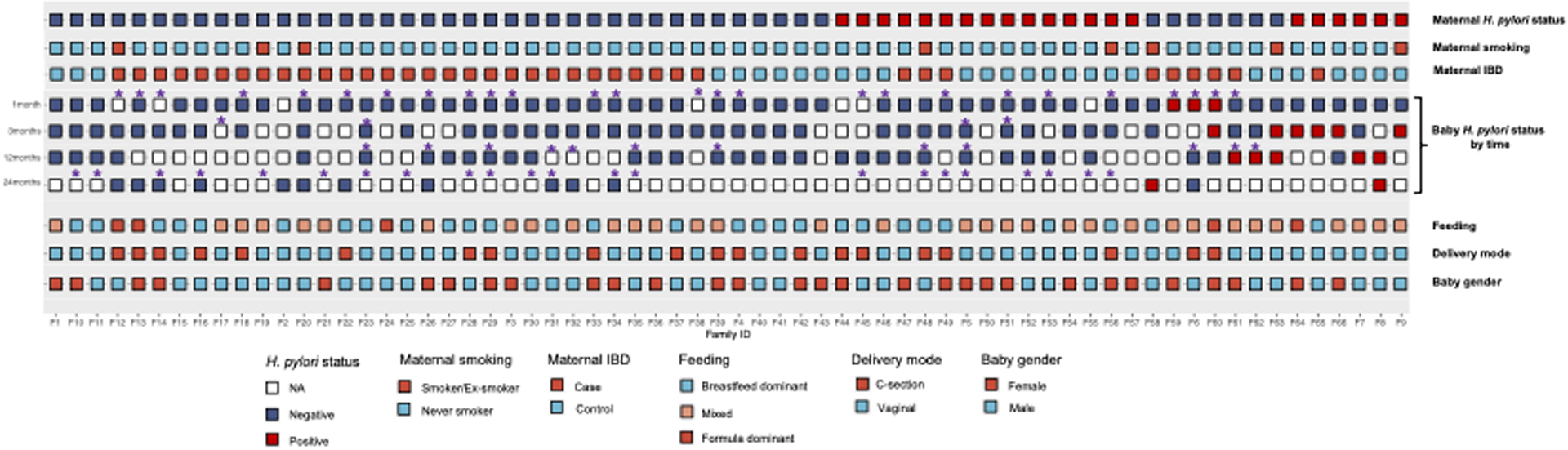
H. pylori status in 66 mothers and their babies at 1 month, 3 months, 12 months, and 24 months along with maternal and baby clinical variables.
The majority of babies tested positive for H. pylori between 3 and 12 months of age. Compared to H. pylori negative babies (28%; n=15/53), H. pylori positive babies more often had H. pylori positive mothers (42%; n= 6/13), albeit not statistically significant (p=0.32). Babies who tested positive vs. negative were less often non-Hispanic white race/ethnicity and less often dominantly breastfed (p-value <0.05). Other clinically observed differences included maternal IBD status, delivery mode and daycare attendance between H. pylori positive and negative babies, but these were not statistically significant (Table 1). Growth metrics (i.e. gestational age, birth weight, head circumference, length) were similar irrespective of babies’ H. pylori status.
On multivariable analysis, adjusted for covariates including maternal H. pylori status, dominant breastfeeding (vs. dominant formula or mixed feeding) (adjusted OR (aOR) 0.17; 95% CI, 0.03–0.98, p=0.049) and maternal IBD (vs. no IBD) diagnosis (aOR 0.05, 95% CI 0.01–0.27, p<0.001) and were associated with significantly lower likelihood of H. pylori colonization in their babies.
Clinical variables for the 38 mothers with IBD, based on H. pylori positive vs. negative status, are provided in Supplementary Table 1. Irrespective of H. pylori status, the majority of patients with IBD were in disease remission.
Mapping of H. pylori status within baby-mother dyads and among other family members
The H. pylori status (positive vs negative) among 66 mothers and their babies at different time points—1 month, 3 months, 12 months, and 24 months—are illustrated in Figure 1. Three infants tested positive at 1 month, all of whom had H. pylori negative mothers, and two of these infants subsequently tested negative at 12 months; no subsequent follow-up information was available for the third baby. Six infants tested positive at 3 months with two out of six (33%) subsequently testing negative at 12 months; no subsequent follow-up information was available for other babies. Of babies tested at 12 months, 5 tested positive for H. pylori, of which 2 had H. pylori positive mothers. A total of 14 babies had fecal samples at 24 months, 2 of whom tested positive for H. pylori (Figure 1). Of these 2, one baby (“F8”) had an H. pylori positive mother, while the other baby’s mother tested negative; notably, the latter baby tested negative at 1 and 3 months of age. Interval antibiotic usage was recorded (Figure 1).
To expand on our findings, we also evaluated stool samples from other family members as available. Fecal samples from other family members were available for 11 distinct families, including 8 fathers and 12 siblings (Figure 2). Seven of 8 fathers tested negative for H. pylori. The frequency of H. pylori positivity among siblings was significantly higher when the mother and the proband infant were both H. pylori positive compared to when only the mother was H. pylori positive. In the only family where both parents were H. pylori positive, both offspring also tested positive. In a family with 6 siblings and a mother who was H. pylori positive, 5 of 6 children also tested positive. There was a mix of H. pylori positive and negative offspring among the 3 families where the proband infant had tested positive for H. pylori.
Figure 2. Mapping of H. pylori status within families.
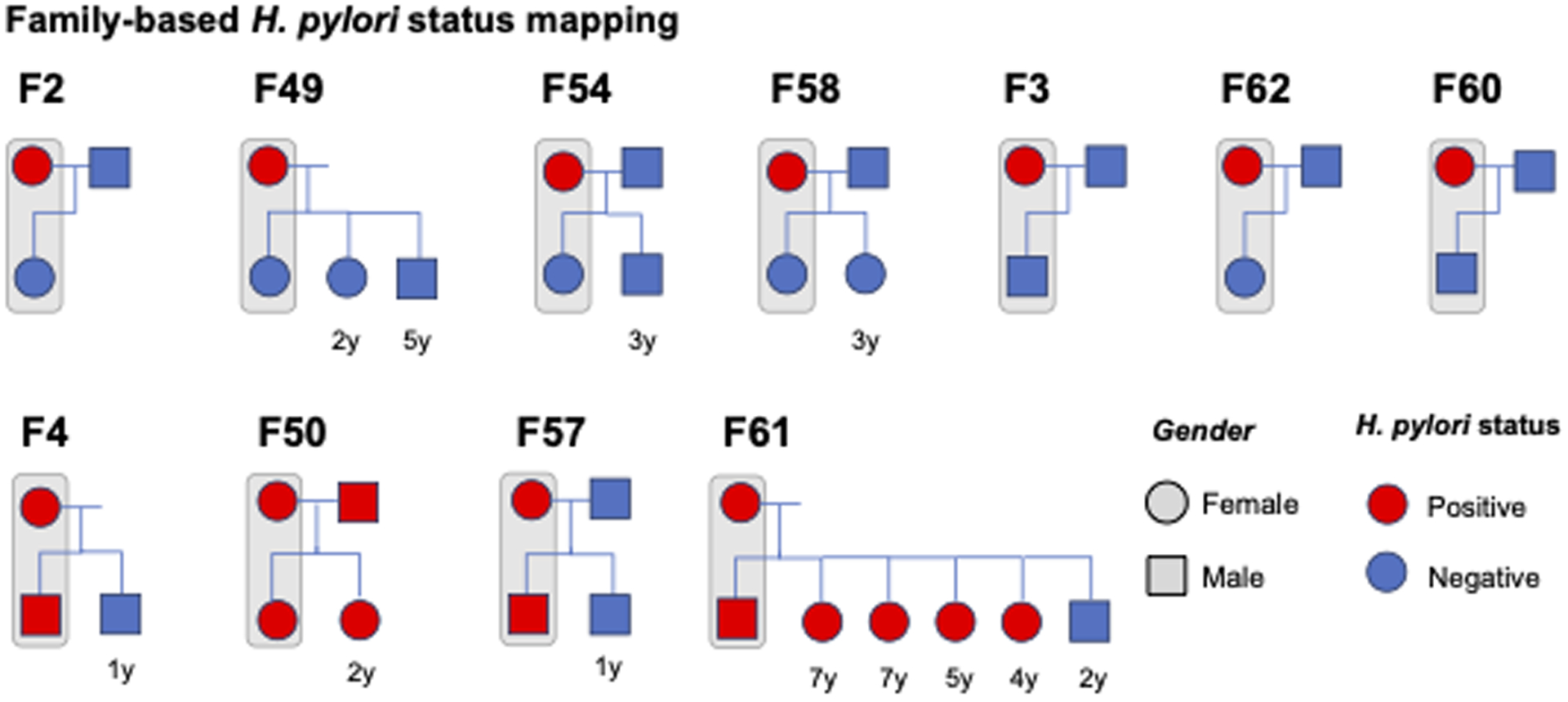
Eleven distinct families had fecal samples and additional information available for other first-degree related household members (fathers, siblings). The results of H. pylori testing by family is depicted. “F” refers to “Family”, while the associated number corresponds to the numeric mother-baby dyad of the 66 total dyads including in the study.
Breastmilk cytokine features associated with baby H. pylori status
The non-supervised PCA analysis demonstrated no significant differences in the cytokine expression profile of maternal breastmilk between mothers with H. pylori positive vs negative babies (p=0.49, Figure 3A). We next used the PLS-DA supervised clustering model to select cytokines associated with H. pylori status in infants. Using this approach, we observed differential clustering by H. pylori status (p=0.002 by PERMANOVA test, Figure 3B). Based on the threshold VIP score >1.5 we identified 14 cytokines that were significantly different between H. pylori positive and negative baby samples (Supplementary Table 2, Figure 3C). Matrix metalloproteinase-10 (MMP10), TNF-related activation-induced cytokine (TRANCE), and C-X-C motif chemokine ligand 10 (CXCL10) were the top 3 cytokines differentially expressed in the breastmilk of mothers with H. pylori positive vs H. pylori negative babies (VIP >2; Supplementary Table 2). After adjusting for clinical variables of maternal H. pylori status, maternal IBD status, delivery mode, feeding type and infant antibiotic usage, and adjusting for multiple testing, MMP10 and TRANCE were still significantly associated with infant H. pylori status (Supplementary Table 2). For every 1 unit increase in expression of MMP or TRANCE, there was an adjusted 2.88-fold (95% CI 1.06–7.81) and 1.22-fold (95% CI 1.00–1.50) higher odds, respectively, of H. pylori colonization in breastfed babies.
Figure 3. Maternal breastmilk cytokines differentially expressed in mothers of H. pylori positive vs negative babies, based on PLS-DA supervised clustering model.
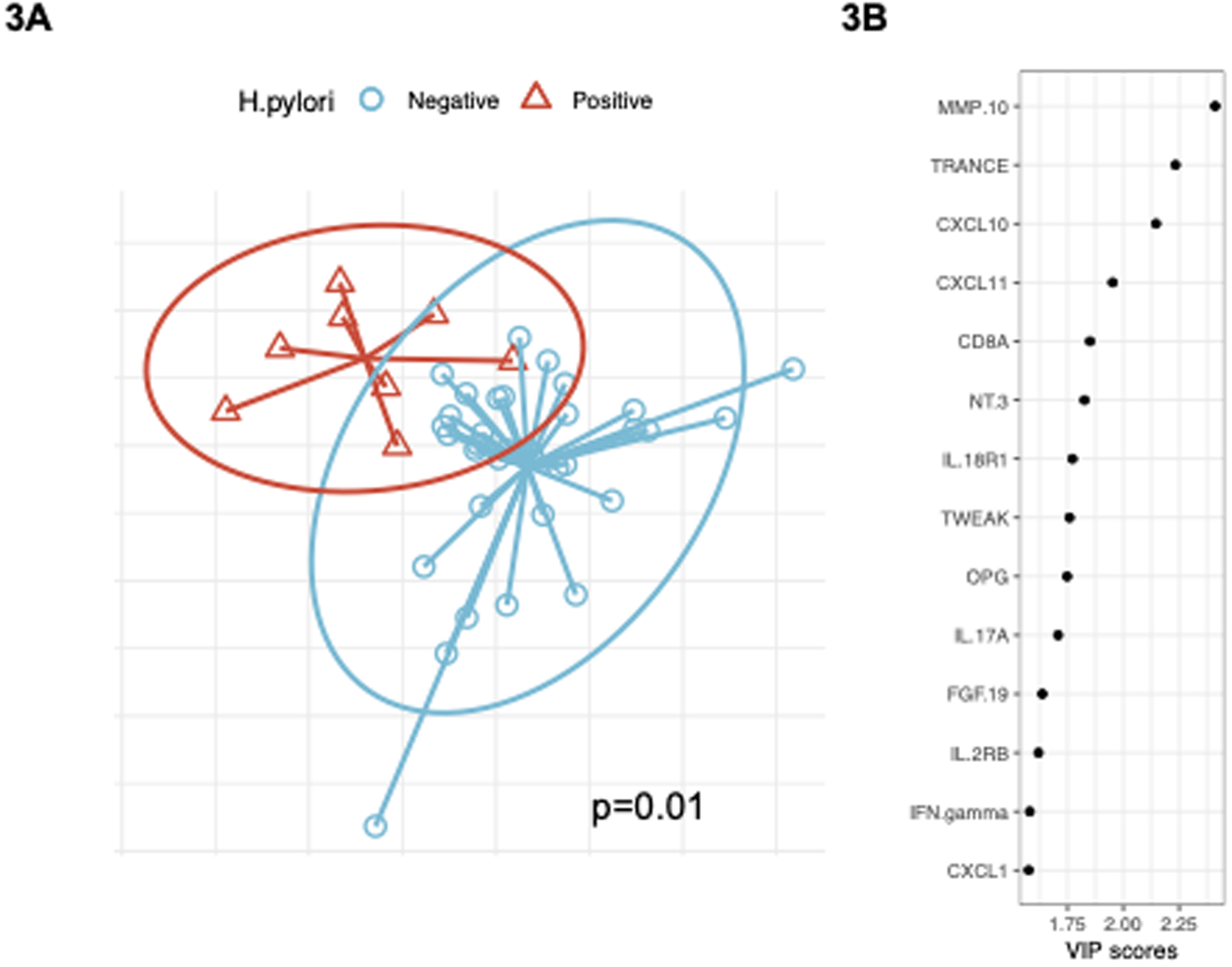
PLS-DA supervised clustering model was used to select the most differentially expressed maternal breastmilk cytokines between H. pylori positive vs. negative breastfed babies. Figure 3A. Supervised PLS-DA analysis demonstrated significantly differential clustering by H. pylori status (p-value=0.01). Figure 3B. A total of 14 cytokines were significantly differentially expressed, as determined based on VIP scores>1.5 (see text).
DISCUSSION
In this US-based study of subjects enrolled in the MECONIUM prospective cohort, we observed that H. pylori colonization among babies is rare in the first month postpartum, and more often occurs in later months. Our findings further suggest that H. pylori infection in babies during the first 12 months postpartum might be transient. We confirmed that dominant breastfeeding (vs mixed or dominant formula feeding) was associated with reduced likelihood of H. pylori colonization in babies, even after adjusting for maternal H. pylori status and other clinical variables, which is consistent with findings from countries where H. pylori infection is endemic. Higher expression of MMP10 and TRANCE cytokines in maternal breastmilk was positively associated with H. pylori colonization in breastfed babies, even after adjusting for relevant covariates and multiple testing, suggesting that these cytokine pathways might mediate risk for colonization. The estimated prevalence of H. pylori in the US is about 35%20,21, although prevalence may be higher or lower depending on factors such as birth cohort and race/ethnicity. The 31.8% observed H. pylori prevalence in this cohort of pregnant mothers aligns with these estimates. Notably, because of the characteristics of the MECONIUM cohort, we were also able to evaluate the potential influence of maternal IBD status on maternal H. pylori colonization. In both epidemiological and experimental studies, H. pylori status has been associated with reduced likelihood of IBD, which is consistent with our findings that H. pylori positive versus negative mothers in this cohort less often had IBD. Moreover, among 38 IBD cases, we did not observe a significant association between maternal H. pylori status and the IBD type, disease activity or administration of IBD-related medications (Supplementary Table 1).
To our knowledge, this is the first study to analyze H. pylori acquisition among newborns and infants in a US population. Despite the formal discovery of H. pylori nearly four decades ago, the exact mode of transmission is still debated. Transmission is thought to occur primarily from person-to-person, although water-borne transmission and other routes have been described.22 The natural history of H. pylori infection, particularly very early or early in life with respect to persistence of infection, is still also poorly understood. Intrafamilial spread is thought to be the primary source of early-in-life H. pylori acquisition. While spread of H. pylori can occur from any positive family member, maternal infection is likely the main source during this early life period.23 In support, one analysis of 1,066 healthy newborns in the Ulm Birth Cohort Study reported that while maternal, paternal, and sibling infection were all significantly associated with risk of infection in the proband child, only maternal infection remained significant in multivariable analysis.10 Very few studies have analyzed H. pylori acquisition in the newborn and early infant period. One study from Japan found that among 51 children born to H. pylori positive mothers, none tested positive by stool antigen testing in the first year of life and only after 1 year did five children test positive (11%).24 Most of the infants in that study were dominantly breastfed during the first year of life, which was hypothesized by the authors to be one reason for the lack of H. pylori infection in this time period. The peak period of infection at least in high prevalence countries is between age 1–5 years, and is also largely attributed to maternal transmission.24–27 However, as noted, these studies did not analyze the very early life period. Moreover, some studies reported only serological H. pylori status in the child and did not confirm the presence of active H. pylori infection.7 This is distinctly relevant, since the transfer of maternal H. pylori antibodies across the placenta and via breastmilk is previously demonstrated.27 The present study confirms that H. pylori acquisition does occur in the first year of life, particularly in the 3–12 month range. The natural history of H. pylori infection acquired this early in life and the clinical implications remains to be determined, particularly given our observation that spontaneous resolution of H. pylori also occurs early in life.
While data from studies conducted in populations of high H. pylori prevalence are generally consistent in demonstrating that breastfeeding is inversely associated with H. pylori infection in early childhood, data from industrialized countries are mixed. One study among 946 children from Germany suggested that breastfeeding in infancy does not protect against H. pylori colonization at least in the pre-school years.8 However, a study from the US among 356 pre-school and school-aged children reported that breastfeeding was strongly protective against H. pylori infection.6 Indeed, our data are consistent with the latter study in the US. Breastmilk contains various bioactive components including essential microbes, human milk oligosaccharides (HMOs), immunoglobulins, lactoferrin and dietary polyunsaturated fatty acids. They play essential roles in immune system development in infants.28 Several mechanisms have been proposed as to why breastfeeding is inversely associated with H. pylori infection24,29,30, at least early in life. Infants breastfed by mothers with higher IgA levels in their breast milk had later H. pylori acquisition compared to infants breastfed by mothers with lower IgA levels in their breastmilk, suggesting IgA as a mediator. Another mechanism, and one that is independent of maternal H. pylori status, is that breastmilk HMOs, in particular, sialic acid-containing oligosaccharides, inhibits H. pylori adherence to gastric mucosal cells.31 Because mothers from non-endemic countries who are infected with H. pylori, as in the present study, possibly have lower levels of H. pylori IgA compared to mothers in endemic countries, it is possible that other mechanisms might be more relevant.31
Among all breastfed mother-baby pairs, we identified significant differential expression of certain key cytokines in maternal breastmilk according to babies’ H. pylori status, suggesting that cytokine alterations in the breastmilk might impact susceptibility of babies to H. pylori colonization. We demonstrated that key features in breastmilk cytokines according to H. pylori status included MMP-10, TRANCE, CXCL10, among others, but that only MMP-10 and TRANCE were statistically significant on multivariable analysis. Studies have demonstrated that MMP-10 is elevated in gastric mucosa and is produced by gastric epithelial cells in the setting of H. pylori colonization, with MMP-10 production synergistically induced by IL-22 via the ERK pathway.32 Human gastric MMP-10 is positively correlated with severity of gastritis in the setting of H. pylori colonization.33 Studies of MMP-10 knockout mice demonstrate decreased likelihood of H. pylori colonization in gastric mucosa. Furthermore, animal models of H. pylori infection demonstrate that cytotoxic CD8+ T cell responses are also relevant for mediating H. pylori colonization.34 Collectively these data suggest that enrichment of MMP-10 and other proinflammatory cytokines in breastmilk may play a role in impairing host defense and increase susceptibility to H. pylori colonization among babies.
In addition to factors associated with H. pylori acquisition in the very early life period, we also investigated whether H. pylori infection in the newborn/infant impacted growth metrics. We found no difference in head circumference, length, nor birthweight in babies with vs without H. pylori infection. We are unable to comment on growth metrics past 1 year, although a handful of studies from developing countries have demonstrated that H. pylori infection in childhood is associated with delayed growth in older children35–37, although residual confounding related to socioeconomic status and other relevant factors might be certainly be at play. This underscores the clinical importance of better defining the natural history of H. pylori infection in very early and early childhood, along with modulating factors and the short- and long-term clinical implications.
Our study has several strengths, including novelty, as it is the first study in a nonendemic population to analyze H. pylori acquisition and persistence in the very early life period, with multiple time points included within the first year of life, and several relevant factors adjusted for (e.g. maternal H. pylori status, day care exposure, antibiotic exposure, type of feeding, mode of delivery and gestational age). We also analyzed associated breastmilk cytokine profiles. Notwithstanding, there are limitations. Firstly, this study included a relatively small sample size since mother-baby pairs were needed, and lacked most follow-up stool samples past 12 months. As such, our analysis is subject to type II error, and some reported null findings reflect insufficient power to detect true differences that may actually exist. Secondly, we had limited availability of paternal and other siblings’ stool samples. Thirdly, we were not able to confirm H. pylori strain congruency between mothers and their offspring due to the low H. pylori load in frozen fecal samples. We acknowledge the possibility that some of the transient infections observed in this study may instead represent false positive or negative tests, although this is presumed to be rare given the very high accuracy of the H. pylori ELISA assay, and also our exclusion of mothers on PPIs, which can reduce the sensitivity of the test.38 Most mothers were recruited from New York City, which may affect generalizability to other US populations and may also impact H. pylori prevalence. We also did not have access to the immigration status and the travel history to endemic H. pylori regions. Lastly, while not a limitation per se, this cohort included 38 women with IBD (30 in disease remission); given the inverse association between H. pylori and IBD39, H. pylori colonization might also be underrepresented in this cohort.
In conclusion, our study confirms that H. pylori acquisition does indeed occur early in life even in regions where H. pylori infection is not endemic, although spontaneous clearance of infection also occurs. Dominant breastfeeding appears to be associated with a lower likelihood of H. pylori acquisition in this early period. We further observed measurable changes in the breastmilk cytokines that may impact H. pylori susceptibility in the breastfed babies. Determining the permanence of these changes and the associated health and immunomodulatory implications are areas in need of focused investigations.
Supplementary Material
Footnotes
Conflict of Interest: The authors have no conflicts of interest or disclosures relevant to this manuscript.
References
- 1.Hooi JKY, Lai WY, Ng WK, et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology. Published online 2017. doi: 10.1053/j.gastro.2017.04.022 [DOI] [PubMed] [Google Scholar]
- 2.Amedei A, Codolo G, Del Prete G, de Bernard M, D’Elios MM. The effect of Helicobacter pylori on asthma and allergy. J Asthma Allergy. Published online 2010. doi: 10.2147/JAA.S8971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Papamichael K, Konstantopoulos P, Mantzaris GJ. Helicobacter pylori infection and inflammatory bowel disease: is there a link? World J Gastroenterol. 2014;20(21):6374–6385. doi: 10.3748/wjg.v20.i21.6374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shah SC, Tepler A, Peek RM, Colombel JF, Hirano I, Narula N. Association Between Helicobacter pylori Exposure and Decreased Odds of Eosinophilic Esophagitis—A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. Published online 2019. doi: 10.1016/j.cgh.2019.01.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gold BD. New approaches to Helicobacter pylori infection in children. Curr Gastroenterol Rep. 2001;3(3):235–247. doi: 10.1007/s11894-001-0028-1 [DOI] [PubMed] [Google Scholar]
- 6.Malaty HM, Logan ND, Graham DY, Ramchatesingh JE. Helicobacter pylori infection in preschool and school-aged minority children: Effect of socioeconomic indicators and breast-feeding practices. Clin Infect Dis. Published online 2001. doi: 10.1086/320148 [DOI] [PubMed] [Google Scholar]
- 7.Malaty HM, El-Kasabany A, Graham DY, et al. Age at acquisition of Helicobacter pylori infection: A follow-up study from infancy to adulthood. Lancet. Published online 2002. doi: 10.1016/S0140-6736(02)08025-X [DOI] [PubMed] [Google Scholar]
- 8.Rothenbacher D, Bodeb G, Brenner H. History of breastfeeding and Helicobacter pylori infection in pre-school children: Results of a population-based study from Germany. Int J Epidemiol. Published online 2002. doi: 10.1093/ije/31.3.632 [DOI] [PubMed] [Google Scholar]
- 9.Weyermann M, Borowski C, Bode G, et al. Helicobacter pylori-specific immune response in maternal serum, cord blood, and human milk among mothers with and without current Helicobacter pylori infection. Pediatr Res. Published online 2005. doi: 10.1203/01.PDR.0000181370.67474.FD [DOI] [PubMed] [Google Scholar]
- 10.Weyermann M, Rothenbacher D, Brenner H. Acquisition of helicobacter pylori infection in early childhood: Independent contributions of infected mothers, fathers, and siblings. Am J Gastroenterol. Published online 2009. doi: 10.1038/ajg.2008.61 [DOI] [PubMed] [Google Scholar]
- 11.Chak E, Rutherford GW, Steinmaus C. The role of breast-feeding in the prevention of Helicobacter pylori infection: A systematic review. Clin Infect Dis. Published online 2009. doi: 10.1086/596499 [DOI] [PubMed] [Google Scholar]
- 12.Okuda M, Miyashiro E, Koike M, Okuda S, Minami K, Yoshikawa N. Breast-feeding prevents Helicobacter pylori infection in early childhood. Pediatr Int. Published online 2001. doi: 10.1046/j.1442-200X.2001.01481.x [DOI] [PubMed] [Google Scholar]
- 13.Peter I, Maldonado-Contreras A, Eisele C, et al. A dietary intervention to improve the microbiome composition of pregnant women with Crohn’s disease and their offspring: The MELODY (Modulating Early Life Microbiome through Dietary Intervention in Pregnancy) trial design. Contemp Clin Trials Commun. Published online 2020. doi: 10.1016/j.conctc.2020.100573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Assarsson E, Lundberg M, Holmquist G, et al. Homogenous 96-plex PEA immunoassay exhibiting high sensitivity, specificity, and excellent scalability. PLoS One. Published online 2014. doi: 10.1371/journal.pone.0095192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fredriksson S, Gullberg M, Jarvius J, et al. Protein detection using proximity-dependent DNA ligation assays. Nat Biotechnol. Published online 2002. doi: 10.1038/nbt0502-473 [DOI] [PubMed] [Google Scholar]
- 16.Huang J, Khademi M, Fugger L, et al. Inflammation-related plasma and CSF biomarkers for multiple sclerosis. Proc Natl Acad Sci U S A. Published online 2020. doi: 10.1073/pnas.1912839117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Santaella A, Kuiperij HB, Van Rumund A, et al. Inflammation biomarker discovery in Parkinson’s disease and atypical parkinsonisms. BMC Neurol. Published online 2020. doi: 10.1186/s12883-020-1608-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiu CY, Yeh KW, Lin G, et al. Metabolomics reveals dynamic metabolic changes associated with age in early childhood. PLoS One. Published online 2016. doi: 10.1371/journal.pone.0149823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monteleone P, Monteleone AM, Troisi J, et al. Metabolomics signatures of acutely ill and short-term weight recovered women with anorexia nervosa. Mol Psychiatry. Published online 2019. doi: 10.1038/s41380-019-0573-3 [DOI] [PubMed] [Google Scholar]
- 20.Perez-Perez GI, Olivares AZ, Foo FY, et al. Seroprevalence of Helicobacter pylori in New York City populations originating in East Asia. J Urban Heal. Published online 2005. doi: 10.1093/jurban/jti093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Everhart JE, Kruszon-Moran D, Perez-Perez GI, Tralka TS, McQuillan G. Seroprevalence and ethnic differences in Helicobacter pylori infection among adults in the United States. J Infect Dis. Published online 2000. doi: 10.1086/315384 [DOI] [PubMed] [Google Scholar]
- 22.Malaty HM, Graham DY, Klein DG, Adam EE, Evans DJ. Transmission of helicobacter pylori infection studies in families of healthy individuals. Scand J Gastroenterol. Published online 1991. doi: 10.3109/00365529108996244 [DOI] [PubMed] [Google Scholar]
- 23.AXON ATR. Review article Is Helicobacter pylori transmitted by the gastro‐oral route? Aliment Pharmacol Ther. Published online 1995. doi: 10.1111/j.1365-2036.1995.tb00426.x [DOI] [PubMed] [Google Scholar]
- 24.Konno M, Fujii N, Yokota SI, et al. Five-year follow-up study of mother-to-child transmission of Helicobacter pylori infection detected by a random amplified polymorphic DNA fingerprinting method. J Clin Microbiol. Published online 2005. doi: 10.1128/JCM.43.5.2246-2250.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ashorn M, Mäki M, Hällström M, et al. Helicobacter pylori infection in finnish children and adolescents a serologic Cross-sectional and follow-up study. Scand J Gastroenterol. Published online 1995. doi: 10.3109/00365529509101594 [DOI] [PubMed] [Google Scholar]
- 26.Rotbenbacher D, Inceoglu J, Bode G, Brenner H. Acquisition of Helicobacter pylori infection in a high-risk population occurs within the first 2 years of life. J Pediatr. Published online 2000. doi: 10.1016/s0022-3476(00)77103-4 [DOI] [PubMed] [Google Scholar]
- 27.Kienesberger S, Perez-Perez GI, Olivares AZ, et al. When is Helicobacter pylori acquired in populations in developing countries? A birth-cohort study in Bangladeshi children. Gut Microbes. Published online 2018. doi: 10.1080/19490976.2017.1421887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thai JD, Gregory KE. Bioactive factors in human breast milk attenuate intestinal inflammation during early life. Nutrients. Published online 2020. doi: 10.3390/nu12020581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weaver LT. Helicobacter pylori infection, nutrition and growth of West African infants. Trans R Soc Trop Med Hyg. 1995;89(4):347–350. doi: 10.1016/0035-9203(95)90002-0 [DOI] [PubMed] [Google Scholar]
- 30.Thomas JE, Austin S, Dale A, et al. Protection by human milk IgA against Helicobacter pylori infection in infancy. Lancet. Published online 1993. doi: 10.1016/0140-6736(93)91327-I [DOI] [PubMed] [Google Scholar]
- 31.Clyne M, Thomas J, Weaver L, Drumm B. In vitro evaluation of the role of antibodies against Helicobacter pylori in inhibiting adherence of the organism to gastric cells. Gut. Published online 1997. doi: 10.1136/gut.40.6.731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Costa AM, Ferreira RM, Pinto-Ribeiro I, et al. Helicobacter pylori activates matrix metalloproteinase 10 in gastric epithelial cells via EGFR and ERK-mediated pathways. J Infect Dis. Published online 2016. doi: 10.1093/infdis/jiw031 [DOI] [PubMed] [Google Scholar]
- 33.pin Lv Y, Cheng P, yu Zhang J, et al. Helicobacter pylori-induced matrix metallopeptidase-10 promotes gastric bacterial colonization and gastritis. Sci Adv. Published online 2019. doi: 10.1126/sciadv.aau6547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kronsteiner B, Bassaganya-Riera J, Philipson N, Hontecillas R. Novel insights on the role of CD8+ T cells and cytotoxic responses during Helicobacter pylori infection. Gut Microbes. Published online 2014. doi: 10.4161/gmic.28899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perri F, Pastore M, Leandro G, et al. Helicobacter pylori infection and growth delay in older children. Arch Dis Child. Published online 1997. doi: 10.1136/adc.77.1.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thomas JE, Dale A, Bunn JEG, et al. Early Helicobacter pylori colonisation: The association with growth faltering in The Gambia. Arch Dis Child. Published online 2004. doi: 10.1136/adc.2002.015313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fall CHD, Goggin PM, Hawtin P, Fine D, Duggleby S. Growth in infancy, infant feeding, childhood living conditions, and Helicobacter pylori infection at age 70. Arch Dis Child. Published online 1997. doi: 10.1136/adc.77.4.310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.George S, Mamani N, Lucero Y, et al. Detection of Helicobacter pylori by Real-Time PCR for 16s rRNA in Stools of NonInfected Healthy Children, Using ELISA Antigen Stool Test as the Gold Standard. Helicobacter. Published online 2016. doi: 10.1111/hel.12318 [DOI] [PubMed] [Google Scholar]
- 39.Tepler A, Narula N, Peek RM, et al. Systematic review with meta-analysis: association between Helicobacter pylori CagA seropositivity and odds of inflammatory bowel disease. Aliment Pharmacol Ther. Published online 2019. doi: 10.1111/apt.15306 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


