Abstract
The emergence of different variants of concern of severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has resulted in upsurges of coronavirus disease 2019 (COVID‐19) cases around the globe. Pakistan faced the fourth wave of COVID‐19 from July to August 2021 with 314,786 cases. To understand the genomic diversity of circulating SARS‐CoV‐2 strains during the fourth wave of the pandemic in Pakistan, this study was conducted. The samples from 140 COVID‐19‐positive patients were subjected to whole‐genome sequencing using the iSeq Sequencer by Illumina. The results showed that 97% (n = 136) of isolates belonged to the delta variant while three isolates belonged to alpha and only one isolate belonged to the beta variant. Among delta variant cases, 20.5% (n = 28) isolates were showing B.1.617.2 while 23.5% (n = 25), 17.59% (n = 19), 14.81% (n = 16), and 13.89% (n = 15) of isolates were showing AY.108, AY.43 AY.127, and AY.125 lineages, respectively. Islamabad was found to be the most affected city with 65% (n = 89) of delta variant cases, followed by Karachi (17%, n = 23), and Rawalpindi (10%, n = 14). Apart from the characteristic spike mutations (T19R, L452R, T478K, P681R, and D950N) of the delta variant, the sublineages exhibited other spike mutations as E156del, G142D, T95I, A222V, G446V, K529N, N532S, Q613H, and V483A. The phylogenetic analysis revealed the introductions from Singapore, the United Kingdom, and Germany. This study highlights the circulation of delta variants (B.1.617.2 and sublineages) during the fourth wave of pandemic in Pakistan.
Keywords: genomic surveillance, Pakistan, SARS‐CoV‐2, variants of concern
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), the causative agent of coronavirus disease 2019 (COVID‐19), belongs to the family of coronaviridae. It is a positive‐sense RNA virus, possessing a large genome (30 kb) with characteristics of accumulating mutations, 1 , 2 which have resulted in the emergence of diverse SARS‐CoV‐2 lineages. 3 , 4 Until September 2021, the genomic surveillance revealed the detection of four variants of concern (VOCs), alpha, beta, gamma, and delta, having increased transmissibility or implications on disease severity, reduced antibody neutralization, therapeutic or vaccination efficacy, or diagnostics. 5 Among the VOCs, alpha was first detected in September 2020, followed by beta in May 2020, gamma in November 2020, and the delta variant in October 2020, which were initially identified in the United Kingdom, South Africa, Brazil, and India, respectively. 6
These VOCs accumulated a considerable number of mutations among which 80% occur in spike glycoprotein which binds to the angiotensin‐converting enzyme 2 (ACE2) receptor. 7 , 8 Among various lineages, alpha showed more mutations and transmissibility than the preceding wild‐type lineages. The beta and gamma also showed higher transmissibility and reinfection rates as compared with the original virus. 9 Delta is suspected of being associated with an increase in cases due to a 60% higher transmissibility rate than alpha, resulting in a higher hospitalization rate. 10 , 11 This may be due to the presence of L452R and T478K spike mutations that increase the RBD‐ACE2 binding free energy by 0.55 and 1.00 kcal/mol, 12 respectively, promoting viral replication, infectivity, and fusogenicity. 13 Moreover, P681H/R found in delta has also been associated with increased virus transmission. 14
In Pakistan, the first SARS‐CoV‐2 case was reported on February 26, 2020, 15 and as of November 2, 2021, it had resulted in 1,274,578 infection cases and 28,477 deaths. Due to persistent virus local transmission, Sindh has the most cases with 470,690 infections, followed by Punjab (n = 440,542), KP (n = 178,204), and Baluchistan (n = 33,274). While Islamabad which is Pakistan's capital has also reported a high number of cases (n = 106,990). 16 Genomic epidemiology showed that the first wave of COVID‐19 in Pakistan was dominated by B.1, the second wave by B.1.36 whereas the third wave was caused by B.1.1.7 (alpha). 17
The fourth wave of COVID‐19 in Pakistan began in July 2021 and peaked on August 4, 2021. Despite the high positivity rate during the fourth wave, data on the circulating SARS‐CoV‐2 strains are limited. Tracking the evolution of variants through time can have a significant impact on disease prevention, diagnosis, and treatment, allowing the health‐care management system to tackle the disease. Hence, during the fourth wave of the epidemic in Pakistan, whole‐genome sequencing of SARS‐CoV‐2 was undertaken to examine the genetic diversity of the virus.
2. MATERIALS AND METHODS
2.1. Sampling
The oropharyngeal swab specimens of suspected subjects (n = 30,200) were collected based on routine surveillance at the National Institute of Health's Department of Virology in Islamabad.
2.2. RNA extraction and real‐time PCR (RT‐PCR)
Total RNA was extracted from the specimens using KingFisherTM Flex Purification System (ThermoFisher Scientific). Clinical RT‐PCR testing was performed using TaqPathTM COVID‐19 CE‐IVD RT‐PCR kit (ThermoFisher Scientific) that targets three genes (ORF1ab, N, and S) was used. The positive samples were subjected to genotyping using SNPsig® SARS‐CoV‐2 (EscapePLEX) kit. This kit was used for the PCR‐based identification of E484K, K417N, K417T, and P681R variant mutations that are characteristics of beta, gamma, and delta variants of concern. Based on genotyping results, a subset of samples was selected for whole‐genome sequencing.
2.3. Next generation sequencing
The paired‐end NGS library (2 × 150 bp) was prepared using the Illumina Ampliseq™ Library Plus (Illumina, Inc) and AmpliSeq™ for Illumina SARS‐CoV‐2 Research Panel (Illumina, Inc) according to the manufacturer's instructions. The prepared libraries were pooled and subjected to sequencing on Illumina platform, iSeq using sequencing reagent, iSeq. 100 i1 Reagent v2 (300‐cycle) (Illumina, Inc).
2.4. Data analysis
The Fastq files were processed for the quality assessment using the FastQC tool (v0.11.9). 18 Trimmomatic (v0.39) 19 was employed to eliminate artifacts and technical biases by removing adapter sequences and low‐quality base calls (< 30). The filtered reads were aligned using the Burrows‐Wheeler Aligner's (BWA, v0.7.17) 20 and available reference genome (Wuhan‐Hu‐1, GISAID ID: EPI_ISL_402125). According to Centers for Disease Control and Prevention (CDC) guidelines, variants were identified and consensus sequences for all genomes were generated. 21 Pangolin v3.1.16 and the pangoLEARN model dated November 04, 2021 were used to classify the assembled genomes into PANGO lineages. 22
2.5. Phylogenetic analysis
The phylogenetic analysis was performed using Nextstrain's standard protocol for analyzing SARS‐CoV‐2 genomes. To begin, BLAST searches against all SARS‐CoV‐2 genome sequences for each of the current study's delta, alpha, and beta isolates were performed on the GISAID database. This resulted in a total of 255 sequences including the sequences from the current study. Augur, Nextstrain's phylodynamic pipeline, was used to perform phylogenetic analysis. 23 MAFFT v7.470 was used to align the sequences to the Wuhan reference genome. 24 The initial phylogenetic tree was constructed using IQTREE v1.6.12, 25 which implements the Augur tree using the generalized time‐reversible (GTR) model and bootstraps the tree topology to ensure a high degree of confidence in it. The raw tree was rooted using the reference genome Wuhan/Hu‐1/2019 (Retrieved from https://www.epicov.org with GISAID ID: EPI ISL 402125). The tree was further processed using TreeTime v0.8.1 to generate a time‐resolved phylogeny based on maximum likelihood. 26 Auspice was used to visualize the resulting tree.
3. RESULTS
From July 1, 2021 to September 19, 2021, a total of 30,200 samples were tested on RT‐PCR for the presence of SARS‐CoV‐2 using the TaqPathTM COVID‐19 kit (ThermoFisher Scientific). A total of 3749 samples were found to be positive for SARS‐CoV‐2. Among positive samples, 97% (n= 3636) of samples were of delta variant. While only 0.6% (n= 23) and 0.2% (n = 11) of cases were of beta and gamma, respectively. There were 2% (n = 79) of samples that were undetermined (neither delta, gamma, nor beta) by genotyping (Figure 1).
Figure 1.
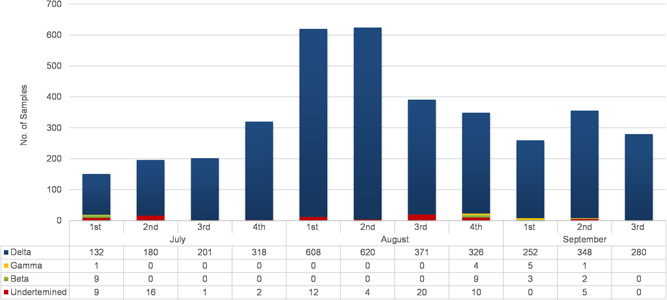
The distribution of SARS‐CoV‐2‐positive patients according to the PCR‐ based genotyping from July 1 to September 19, 2021. The SNPsig® SARS‐CoV‐2 (EscapePLEX) kit was used to detect different VOCs (beta, gamma, and delta) in SARS‐CoV‐2‐positive patients. The X‐axis represents the weeks of the months, while the Y‐axis represents the number of cases. SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2; PCR, polymerase chain reaction.
According to month‐wise distribution, an increasing trend in the number of delta cases has been observed. In July, a total of 869 samples were found to be positive for SARS‐CoV‐2. Among them, 95% (n = 831) of cases were of the delta while the beta cases were only 1% (n = 09). In August 2021, a total of 1984 samples were found to be SARS‐CoV‐2 positive. Among them 97% (n = 1925) of delta variant cases while beta and gamma were found to be <0.5%. The maximum number of delta cases was observed in the 1st and 2nd weeks of August 2021. During the first three weeks of September 2021, 98% (n = 880) of samples were of delta variant. Among the positive cases, a subset (n = 158) of samples were selected for whole‐genome sequencing. Among 158 whole genomes, 140 high‐quality complete SARS‐CoV‐2 genomes with <5% Ns were selected for further analysis.
During the studied period, three lineages, that is, delta, alpha, and beta were identified from Pakistan. Among VOCs, 97.14% (n = 136/140) belonged to delta, 2.14% (n = 03/140) belonged to alpha, and 0.71% (n = 1) comprised of beta variant. A total of 22 sublineages of delta (B.1.617.2, n= 28; 20.58%) were found with AY.108 (n = 25; 23.15%) reported highest in number followed by AY.43 (n = 19; 17.59%), AY.127 (n = 16, 14.81%), AY.125 (n = 15; 13.89%), and AY.103 (n = 6; 5.56%). Other delta sublineages include AY.126, AY.59, AY.111, AY.65, AY.99, AY.62, AY.56, AY.5.4, AY.122, AY.112, AY.88, AY.45, AY.86, AY.4, AY.118, AY.116, and AY.20 (Figure 2). Among delta variant cases, Islamabad reported the highest numbers (n = 89; 65.71%) followed by Karachi (n = 23; 16.91%) and Rawalpindi (n = 14; 10.29%). The other, 7.35% reported regions included Murree (n = 2) and one case each case from Peshawar, Azad Jammu Kashmir (AJK), Multan, Sargodha, Attock, and Panjgur. The two patients (1.47%) infected with the delta variant had been associated with international travel from UAE (GISAID IDS: EPI_ISL_4458425), and Ghana (GISAID ID: EPI_ISL_4458434). Furthermore, the travel history of one male patient from Afghanistan with a beta variant was also reported (GISAID ID: EPI_ISL_4018916).
Figure 2.
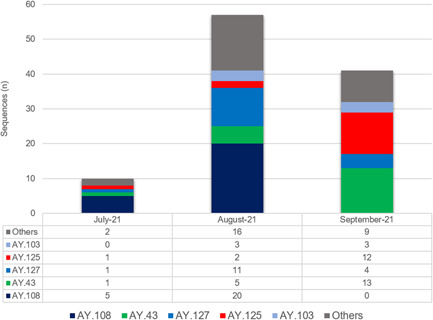
Distribution of sublineages (n = 22) of delta variant
Our data included 61 vaccinated patients with delta variant infection, of which 38 (27.9%) patients were fully vaccinated (received both doses of vaccine) while 23 (16.9%) patients were partially vaccinated (received only one dose of vaccine). Vaccination status showed that 60 (44.1%) patients were unvaccinated and vaccination data of 15 (11.0%) individuals were not available. In the fully vaccinated group, the male‐to‐female ratio was 28:10 while in the partially vaccinated group the ratio was 18:5. The highest rate of vaccination and infection was observed in the age group of 18–49 years. Among the partially vaccinated individuals, 60% had received Sinovac, 30% had received Sinopharm, while 4.3% each received Moderna and AstraZeneca. Among the fully vaccinated group, the individuals received Sinovac (26%), Sinopharm (47%), CanSino (15%), Pakvac, AstraZeneca (2.6% each), and Pfizer (2%) (Table 1).
Table 1.
Characteristics of the study participants by vaccination status
| Unvaccinated, N(%) | Partly vaccinated, N(%) | Fully vaccinated, N(%) | |
|---|---|---|---|
| Total | 60 | 23 | 38 |
| Gender | |||
| Female | 31 (51.6%) | 5 (21.7%) | 10 (26.3%) |
| Male | 29 (48.3%) | 18 (78.2%) | 28 (73.6%) |
| Age | |||
| 0–11 | 14 (23.3%) | NA | NA |
| 12–17 | 4 (6.6%) | 2 (8.6%) | 0 (0%) |
| 18–29 | 16 (26.6%) | 10 (43.4%) | 15 (39.4%) |
| 30–49 | 15 (25.0%) | 6 (26.0%) | 16 (42.1%) |
| 50–74 | 9 (15.0%) | 4 (17.3%) | 6 (15.7%) |
| 75 + | 2 (3.3%) | 0 (0%) | 1 (2.6%) |
| Vaccine received | |||
| Sinovac | NA | 14 (60.8%) | 10 (26.3%) |
| Sinopharm | NA | 7 (30.4%) | 18 (47.3%) |
| Pak‐vac | NA | 0 (0%) | 1 (2.6%) |
| Cansino | NA | 0 (0%) | 6 (15.7%) |
| Pfizer | NA | 0 (0%) | 2 (5.2%) |
| Moderna | NA | 1 (4.3%) | 0 (0%) |
| Astrazanca | NA | 1 (4.3%) | 1 (2.6%) |
We have found a total of 635 nucleotide changes in 136 delta variant isolates out of which, 379 have been the nonsynonymous single nucleotide polymorphisms (SNPs) and 247 have been synonymous SNPs. In addition, a few Pakistani sequences had seven deletions and two stop codons. Among the nonsynonymous SNPs, there were 70 SNPs with >2% frequency.
Observably, the ORF1ab was the most mutated protein with the highest number of mutations in NSP3, NSP2, and NSP12 while NSP5 and NSPs7‐10 were relatively conserved. Membrane, Envelope, ORF6, and ORF10 were found to be the relatively conserved proteins. Interestingly, novel deletions were observed in NSP1 and NSP2 proteins. In NSP1, the deletion was observed at position V84del (GISAID ID: EPI_ISL_4197108), in NSP2 the A80del (GISAID ID: EPI_ISL_4630059). While analyzing the mutation profile we have found characteristic spike mutations (T19R, L452R, T478K, P681R, and D950N) in the delta variant. In addition to these mutations, there were other mutations observed in delta variant as E156del, G142D, T95I, and A222V. Interestingly, some unique mutations were also observed in the spike protein as G446V, K529N, N532S, Q613H, and V483A. In ORF7a and ORF7b, stop codon has been observed at position 94 as Q94* (GISAID ID: EPI_ISL_3827532) and position 39 as E39* (GISAID ID: EPI_ISL_4018954). A unique deletion was also observed in ORF7a and ORF7b as F65del (GISAID ID: EPI_ISL_4630058) and F13del (GISAID ID: EPI_ISL_4458437), respectively.
Other than the delta variant the subset of studied samples also revealed 03 alpha and 01 beta cases. The characteristic spike mutations observed in alpha have been H69del, Y145del, N501Y, A570D, P681H, T716I, S982A, and D1118H and in the beta SARS‐CoV‐2 variant, it has been D80A, D215G, L242del, K417N, E484K, N501Y, and A701V.
To infer the origin of Pakistani isolates, we built a maximum likelihood phylogenetic tree using 255 full‐length genomes of SARS‐CoV‐2 viruses including the current study's high‐quality genomes (n = 140/158). In the phylogenetic tree, the VOCs detected in the current study showed close similarities with isolates originating from Asia, Europe, and North America. The beta‐variant infected patient (GISAID ID: EPI_ISL_4018916) has a travel history to Afghanistan, clustered mostly with United Kingdom viral isolates. The delta variant GISAID ID: EPI_ISL_4458434 (having a travel history of Ghana) clustered with viruses from India and Germany. Moreover, delta variant EPI_ISL_4458425 isolated from a patient with a travel history of Dubai showed close homology with strains from India and Denmark (Figure 3). These results suggest the probable introduction of VOCs through inbound travelers in Pakistan from different countries.
Figure 3.
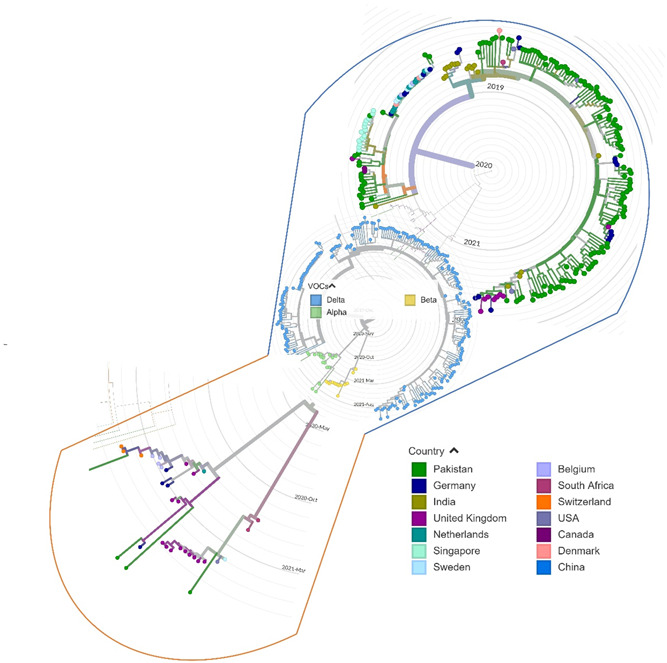
Phylogenetic distribution of alpha, beta, and delta lineages in 255 SARS‐CoV‐2 viral genomes from around the world, including current study sequences from Pakistan with reference to the Wuhan/Hu‐1/2019 (GISAID ID: EPI_ISL_402125). The maximum likelihood phylogenetic tree was constructed using Nextstrain's Augur tree implementation pipeline and the default parameters were used for IQ‐TREE. The time‐resolved phylogenetic tree with selected metadata information was constructed using TreeTime and visualized in Auspice. SARS‐CoV‐2, severe acute respiratory syndrome coronavirus 2.
4. DISCUSSION
Pakistan has reported 1,297,235 laboratory‐confirmed cases of COVID‐19, mainly distributed among four waves of infection (as of January 02, 2022). After confirmation of the first COVID‐19 case from Karachi on February 26, 2020, SARS‐CoV‐2 began spreading to different parts of the country which led to the start of the first wave in April that peaked in June and eventually subsided in July, 2020. Although genomic surveillance was limited at the time, we reported the circulation of a highly transmissible variant of SARS‐CoV‐2 (G614) during June, 2020. 27 The second wave started in October and peaked in December with the highest cases (n = 3795) reported on December 06, 2020. Genomic surveillance revealed the prevalence of several lineages of SARS‐CoV‐2 during the second wave however, B.1.36 was predominant. The third wave of COVID‐19 which spanned over 4 months started in mid‐February and continued till mid‐June 2021 with the highest number of cases (n = 6127) reported on April 17, 2021. Genomic surveillance showed that the third wave was mainly driven by the alpha that contributed to the nationwide spread of the disease. As the country was recovering from the effects of the third wave, Pakistan was hit by another devastating surge of infections during July–October 2021. The fourth wave resulted in 314,786 cases and 6075 deaths that challenged an already depleted health system.
Our results revealed the predominance of delta (B.1.617.2) with the circulation of its sublineages (AY.108, AY.43 AY.127, and AY.125). Although we have found a high percentage of AY.108 cases, very few sequences of this sublineage (n= 862) have been reported globally as compared with AY.43 (n = 296,668), AY.127 (n = 25,048), and AY.125 (n = 29,224) as of February 10, 2022. 28 , 29 , 30 , 31 The delta variant was first reported from India in September 2020, which resulted in the ferocious second wave starting in March 2021, with the highest cases (n = 414,188) reported on May 06, 2021. 32 According to GISAID data, it had spread to 62 countries by June 1, 2021, 2 weeks later, it was reported by 80 countries, and by September 21, 2021, the number had risen to 185 countries. Delta caused a resurgence of COVID‐19 in regional countries such as India, Bangladesh, Singapore, Indonesia, Russia, and Nepal with a cumulative prevalence of more than 95% as of September 5, 2021. Similarly, United Kingdom and United States witnessed a rise in SARS‐CoV‐2 infections during the first 3 weeks of June 2021 and July 2021, respectively, with 99% of cases associated with delta (as of September 6, 2021). 33 The first case of the delta from Pakistan was reported on May 28, 2021, by the National Institute of Health. 34 Since July 2021, a sharp increase in COVID‐19 cases was observed in Pakistan, with the highest cases (n = 7727) reported on September 04, 2021. This rise in COVID‐19 was attributed to the spread of highly transmissible delta, which is in concordance with our sequencing results.
According to the phylogenetic analysis, the three VOCs detected in our study, that is, delta, alpha, and beta showed close homology with viruses from Asia, Europe, and North America. The delta sequences clustered mostly with viruses from Germany, India, Singapore, Denmark, and England. The delta strain EPI_ISL_4458434 isolated from a patient with a travel history of Ghana grouped with viruses from India and Germany whereas EPI_ISL_4458425 was detected from a traveler returning from the United Arab Emirates and showed close association with strains from India and Denmark. Pakistan has implemented a strong inbound air travel protocol with vaccination mandatory before travel, along with a negative COVID‐19 PCR confirmation 48 h before boarding. This also includes airport testing of all passengers coming from Europe and other countries using rapid SARS‐CoV‐2 antigen detection kits. However, genome sequencing of samples from COVID‐19‐positive inbound passengers remains a challenge. Moreover, there is limited diagnostic testing at the land border crossings with Afghanistan and Iran (with a high influx of people entering Pakistan on daily basis) mainly due to insufficient testing facilities in these areas. This however raises a serious concern as the first case of COVID‐19 in February 2020 was introduced through Pakistani travelers coming back from Iran 35 followed by frequent introductions through international travel. 34 , 36 , 37 For the effective control of COVID‐19, Pakistan thus needs to implement mandatory testing along with target sequencing of all inbound travelers entering the country through land and other ports of entry.
The SARS‐CoV‐2 spike protein is a major target for vaccine and drug development due to its association with host cell receptor recognition, attachment, and entry. 38 , 39 Notably, a rare spike deletion, I210del was found in a patient (EPI_ISL_4458434) traveling back from Ghana (I210del mutation has a prevalence of only 0.1% in the delta (as of February 11, 2022). This mutation is found in recurrent deletion region 3 (RDRs), which is known to confer resistance to neutralizing antibodies. 40 Moreover, in one female patient (EPI_ISL_41971110), a rare spike mutation L54F was observed. This mutation, when combined with D614G, resulted in an increase in virulence as measured by its effective binding to the ACE2 receptor, thus increasing infectivity and enhanced transmissibility. 41 In one sequence (EPI_ISL_3462513) of our study, a similar finding had been reported, where the A845S spike mutation (about 0.45% global prevalence) has been proposed to aid in the transmissibility. 42 We have also reported the distinct spike mutations F490S and G446V (EPI_ISL_3462513, EPI_ISL_3462511, EPI_ISL_4630058, and EPI_ISL_4630381) in our study sequences. These mutations in combination with L452R were shown to exhibit resistance to neutralization by polyclonal human immune convalescent sera in some in vitro studies. 43 These findings underscore the vital importance of ongoing monitoring of amino acid alterations in the spike area in the creation of vaccines and therapeutic antibodies. The G142D (33% global prevalence), A22V (8% global prevalence), and T95I (19% global prevalence) are the mutations that lie in the N‐terminal domain of spike protein. It has been reported that the N‐terminal domain of spike protein also helps the viral entry to the host cell and it also aids recognition by viral neutralizing antibodies. 44 , 45 The changes in the NTD should be further evaluated in terms of their effect on virus neutralization. Overall, the mutations in the spike protein need further monitoring in terms of vaccine development and therapeutic antibodies.
Apart from spike protein, we also identified unique mutations in ORF7a, ORF7b, and NSP1 of the study isolates. Two unusual stop codons have been identified in ORF7a and ORF7b as Q94* and E39*, respectively. These two stop codons have not been observed in any of the worldwide reported sequences (as of February 10, 2022). Functionally, the ORF7a protein binds to human monocytes leading to decreasing antigen‐presenting ability and inducing dramatic expression of proinflammatory cytokines. 46 The unusual stop codons result in premature truncation of protein, thus impairing the protein function. The luminal domain of ORF7a (16‐96 amino acids) is involved in binding with human monocytes, hence any variability in this region may affect the binding properties. 47 In the studied isolates, (GISAID ID: EPI_ISL_4197108) another deletion was identified in Nsp1 as V84del and this deletion is 1.3% prevalent in worldwide reported sequences (as of February 10, 2022). The Nsp1 is involved in modulating host immune responses and blocks the host mRNA translation by binding to 40 S ribosomes. 48 The unusual stop codons and deletions identified in the ORF7a, ORF7b, and NSP1 along with unique changes in spike protein in the studied isolates, warrant enhanced genomic surveillance and close monitoring of these changes as there might be a chance of emergence of new variants of SARS‐CoV‐2. These deletions along with other mutations may also participate in viral infection, transmission, and immunomodulation.
Vaccination is an important intervention for the prevention and control of infectious diseases. In Pakistan, the inactivated Chinese vaccines (Sinopharm, Sinovac, and CanSino) are majorly administered and about 11% of the population was fully vaccinated while 24.9% is partially vaccinated (as of September 19, 2021). The real‐world data of vaccine efficacy against delta variant is reported for only a few vaccines. The efficacy of AstraZeneca and Pfizer has been tested against delta variants in the United Kingdom and results showed 59.8% and 87.9% protection, respectively. A study showed that Pfizer provides protection against disease severity and reduces the hospitalization rate to 88%. 49 Apart from these vaccines, that is, AstraZeneca and Pfizer, the inactivated vaccines (Sinopharm, Sinovac, and CanSino, CoVaxin) developed in China and India, also provide protection against delta variant by reducing the disease severity and deaths. 50 The efficacy of these vaccines decreased significantly in comparison to the original strain, which was primarily due to weakened neutralization and the failure of monoclonal antibodies. 51 , 52 , 53 In this study, we also found that 27.9% (n = 38/136) of positive cases are linked to vaccine breakthrough infections with delta variants. In January 2021, breakthrough infections were reported in the population vaccinated with the Pfizer vaccine in Israel and later in India. Immediately afterward, breakthrough infections due to the delta were reported in several countries including the United States, Italy, and the United Kingdom. While it is common for the delta to cause breakthrough infections, vaccination can help avoid severe infection and linked hospitalizations, 54 as it develops memory immunity and is capable of providing long‐lasting protection through memory cells. 55 However, there is a reduction in the receptiveness of memory B cells to VOCs in vaccinated individuals, resulting in the collapse of both memory and humoral immune system and consequently, triggering the spread of breakthrough infections. 56 , 57
The dynamics of the fourth wave of COVID‐19 in Pakistan have shown an intriguing epidemic modifying pattern that is important in national decision‐making for dealing with the pandemic. The decrease in hospitalization even with a highly transmissible variant of concern suggests that vaccination coverage might help in suppressing future waves of the COVID‐19 in the Pakistani population. Furthermore, the disease also highlights the continued effectiveness of genomic surveillance of SARS‐CoV‐2 variants.
AUTHOR CONTRIBUTIONS
Conceptualization: Massab Umair, Aamer Ikram, and Muhammad Salman. Methodology: Muhammad Ammar, Qasim Ali, Syed Adnan Haider. Formal analysis: Syed Adnan Haider, Zaira Rehman, Qasim Ali; resources: Massab Umair, Aamer Ikram, and Muhammad Salman. Writing—original draft preparation: Zaira Rehman, Muhammad Ammar, Nazish Badar and Syed Adnan Haider. Writing—review and editing: Massab Umair, Aamer Ikram, Muhammad Salman, and Muhammad SalmanR. All authors have read and agreed to the published version of the manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Umair M, Ikram A, Rehman Z, et al. Genomic diversity of SARS‐CoV‐2 in Pakistan during the fourth wave of pandemic. J Med Virol. 2022;94:4869‐4877. 10.1002/jmv.27957
DATA AVAILABILITY STATEMENT
All the sequences generated in the current study are submitted to the GISAID that are available at “https://www.gisaid.org/login/” under the accession numbers: EPI_ISL_3462498‐ EPI_ISL_3462527, EPI_ISL_3553499‐EPI_ISL_3553528, EPI_ISL_3827529‐EPI_ISL_3827557.
REFERENCES
- 1. Kim D, Lee JY, Yang JS, Kim JW, Kim VN, Chang H. The architecture of SARS‐CoV‐2 transcriptome. Cell. 2020;181(4):914‐921e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu D, Wu T, Liu Q, Yang Z. The SARS‐CoV‐2 outbreak: what we know. Int J Infect Dis. 2020;94:44‐48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mohammadi M, Shayestehpour M, Mirzaei H. The impact of spike mutated variants of SARS‐CoV2 [alpha, beta, gamma, delta, and lambda] on the efficacy of subunit recombinant vaccines. Braz J Infect Dis. 2021;25:101606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cella E, Benedetti F, Fabris S, et al. SARS‐CoV‐2 lineages and sub‐lineages circulating worldwide: a dynamic overview. Chemotherapy. 2021;66(1‐2):3‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. World Health Organization . Tracking SARS‐CoV‐2 variants. 2022. Accessed November 2, 2021. https://www.who.int/en/activities/tracking-SARS-CoV-2-variants/
- 6. Choi JY, Smith DM. SARS‐CoV‐2 variants of concern. Yonsei Med J. 2021;62(11):961‐968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Callaway E. The coronavirus is mutating–does it matter? Nature. 2020;585(7824):174‐178. [DOI] [PubMed] [Google Scholar]
- 8. Ramanathan M, Ferguson ID, Miao W, Khavari PA. SARS‐CoV‐2 B. 1.1. 7 and B. 1.351 spike variants bind human ACE2 with increased affinity. Lancet Infect Dis. 2021;21(8):1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mandal N, Padhi AK, Rath SL. Molecular insights into the differential dynamics of SARS‐CoV‐2 variants of concern (VOCs). J Mol Graph Model. 2022;114:108194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Duong D. Alpha, beta, delta, gamma: What's important to know about SARS‐CoV‐2 variants of concern? Can Med Assoc. 2021;193(27):E1059‐E1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. outbreak.info Accessed November 3, 2021. https://outbreak.info/situation-reports
- 12. Chen J, Wang R, Wei G‐W. Review of the mechanisms of SARS‐CoV‐2 evolution and transmission. ArXiv. 2021;arXiv:2109.08148v1.
- 13. Motozono C, Toyoda M, Zahradnik J, et al. SARS‐CoV‐2 spike L452R variant evades cellular immunity and increases infectivity. Cell Host Microbe. 2021;29(7):1124‐1136e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu Y, Liu J, Johnson BA, et al. Delta spike P681R mutation enhances SARS‐CoV‐2 fitness over alpha variant. BioRxiv. 2021. [DOI] [PMC free article] [PubMed]
- 15. Jabeen K, Husnain Haider M B, Haider Z, et al. Coronavirus (COVID‐19) pandemic: outbreak, current scenario and impact on human physiology in Pakistan. Global J Clin Virol. 2021;6(1):021‐029. [Google Scholar]
- 16. COVID‐19 . Health advisory platform by Ministry of National Health Services Regulations and Coordination.
- 17. Basheer A, Zahoor I. Genomic epidemiology of SARS‐CoV‐2 divulge B. 1, B. 1.36, and B. 1.1. 7 as the most dominant lineages in first, second, and third wave of SARS‐CoV‐2 infections in Pakistan. Microorganisms. 2021;9(12):2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Andrews S, FastQC: a quality control tool for high throughput sequence data. 2010, Babraham Bioinformatics, Babraham Institute, Cambridge, United Kingdom. [Google Scholar]
- 19. Bolger AM, Lohse M, Usadel B. Trimmomatic: a flexible trimmer for illumina sequence data. Bioinformatics. 2014;30(15):2114‐2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li H, Durbin R. Fast and accurate long‐read alignment with Burrows–Wheeler transform. Bioinformatics. 2010;26(5):589‐595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Paden CR, Tao Y, Queen K, et al. Rapid, sensitive, full‐genome sequencing of severe acute respiratory syndrome coronavirus 2. Emerging Infect Dis. 2020;26(10):2401‐2405. https://wwwnc.cdc.gov/eid/article/26/10/20-1800_article [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. O'Toole A, Scher E, Underwood A, et al. Pangolin: lineage assignment in an emerging pandemic as an epidemiological tool. Virus Evolution. 2021;7(2):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Huddleston J, Hadfield J, Sibley TR, et al. Augur: a bioinformatics toolkit for phylogenetic analyses of human pathogens. J Open Source Softw. 2021;6(57):2906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Katoh K, Misawa K, Kuma K, Miyata T. MAFFT: a novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002;30(14):3059‐3066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nguyen L‐T, Schmidt HA, von Haeseler A, Minh BQ. IQ‐TREE: a fast and effective stochastic algorithm for estimating maximum‐likelihood phylogenies. Mol Biol Evol. 2015;32(1):268‐274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sagulenko P, Puller V, Neher RA. TreeTime: maximum‐likelihood phylodynamic analysis. Virus Evol. 2018;4(1):vex042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Umair M, Ikram A, Salman M, et al. Whole‐genome sequencing of SARS‐CoV‐2 reveals the detection of G614 variant in Pakistan. PLoS One. 2021;16(3):e0248371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Alaa Abdel L, Julia LM, Manar A, et al. AY.108 Lineage Report.outbreak.info. Accessed February 10, 2022. https://outbreak.info/situation-reports?pango=AY.108%26loc=IND%26loc=GBR%26loc=USA%26selected
- 29. Alaa Abdel L, Julia L M, Manar A, et al. AY.125 Lineage Report.outbreak.info. Accessed February 10, 2022. https://outbreak.info/situation-reports?pango=AY.125%26loc=IND%26loc=GBR%26loc=USA%26selected
- 30. Alaa Abdel L, Julia L M, Manar A, et al. AY.43ineage Report. outbreak.info. Accessed February 10, 2022. https://outbreak.info/situation-reports?pango=AY.43%26loc=IND%26loc=GBR%26loc=USA%26selected
- 31. AY. 127 Lineage Report . Alaa Abdel L, Julia L M, Manar A, et al. Hughes, and the Center for Viral Systems Biology. outbreak.info. Accessed February 10, 2022. https://outbreak.info/situation-reports?pango=AY.127%26loc=IND%26loc=GBR%26loc=USA%26selected
- 32. Singh J, Rahman SA, Ehtesham NZ, Hira S, Hasnain SE. SARS‐CoV‐2 variants of concern are emerging in India. Nature Med. 2021;27:1‐3. [DOI] [PubMed] [Google Scholar]
- 33. Shu Y, McCauley J, GISAID: Global initiative on sharing all influenza data–from vision to reality. Eurosurveillance. 2017;22(13):30494. https://www.gisaid.org [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Umair M, Ikram A, Salman M, et al. Genomic surveillance reveals the detection of SARS‐CoV‐2 delta, beta, and gamma VOCs during the third wave in Pakistan. J Med Virol. 2022;94(3):1115‐1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Arab News Pakistan . Pakistan prepares to fight back as two coronavirus cases emerge in country. Accessed February 13, 2021. https://www.arabnews.pk/node/1633656/pakistan
- 36. Umair M, Ikram A, Salman M, et al. Importation of SARS‐CoV‐2 variant B. 1.1. 7 in Pakistan. J Med Virol. 2021;93:2623‐2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tamim S, Trovao NS, Thielen P, et al. Genetic and evolutionary analysis of SARS‐CoV‐2 circulating in the region surrounding Islamabad, Pakistan. Infect Genet Evol. 2021;94:105003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ortega JT, Serrano ML, Pujol FH, Rangel HR. Role of changes in SARS‐CoV‐2 spike protein in the interaction with the human ACE2 receptor: an in silico analysis. EXCLI J. 2020;19:410‐417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Liu Z, Xiao X, Wei X, et al. Composition and divergence of coronavirus spike proteins and host ACE2 receptors predict potential intermediate hosts of SARS‐CoV‐2. J Med Virol. 2020;92(6):595‐601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McCarthy KR, Rennick LJ, Nambulli S, et al. Recurrent deletions in the SARS‐CoV‐2 spike glycoprotein drive antibody escape. Science. 2021;371(6534):1139‐1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Laha S, Chakraborty J, Das S, Manna SK, Biswas S, Chatterjee R. Characterizations of SARS‐CoV‐2 mutational profile, spike protein stability and viral transmission. Infect Genet Evol. 2020;85:104445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Klink GV, Safina K, Nabieva E, et al. Spread of endemic SARS‐CoV‐2 lineages in Russia. medRxiv.2021. [DOI] [PMC free article] [PubMed]
- 43. Liu Z, VanBlargan LA, Bloyet LM, et al. Identification of SARS‐CoV‐2 spike mutations that attenuate monoclonal and serum antibody neutralization. Cell Host Microbe. 2021;29(3):477‐488e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Qing E, Kicmal T, Kumar B, et al. Dynamics of SARS‐CoV‐2 spike proteins in cell entry: control elements in the amino‐terminal domains. mBio. 2021;12(4):e0159021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Di Gaetano S, Capasso D, Delre P, et al. More is always better than one: the N‐terminal domain of the spike protein as another emerging target for hampering the SARS‐CoV‐2 attachment to host cells. Int J Mol Sci. 2021;22(12):6462‐ 6471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zhou Z, Huang C, Zhou Z, et al. Structural insight reveals SARS‐CoV‐2 ORF7a as an immunomodulating factor for human CD14(+) monocytes. iScience. 2021;24(3):102187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Panzera Y, Ramos N, Frabasile S, et al. A deletion in SARS‐CoV‐2 ORF7 identified in COVID‐19 outbreak in Uruguay. Transbound Emerg Dis. 2021;68(6):3075‐3082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Benedetti F, Snyder GA, Giovanetti M, et al. Emerging of a SARS‐CoV‐2 viral strain with a deletion in nsp1. J Transl Med. 2020;18(1):329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nasreen S, Chung H, He S, et al. Effectiveness of COVID‐19 vaccines against variants of concern in Ontario. Nat Microbiol. 2022;7:379‐385. [DOI] [PubMed] [Google Scholar]
- 50. Bian L, Gao Q, Gao F, et al. Impact of the delta variant on vaccine efficacy and response strategies. Expert Rev Vaccines. 2021;20(10):1201‐1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Chaudhari AM, et al. E156G and Arg158, Phe‐157/del mutation in NTD of spike protein in B. 1.617. 2 lineage of SARS‐CoV‐2 leads to immune evasion through antibody escape. BioRxiv. 2021.
- 52. Chen X, Chen Z, Azman AS, et al. Neutralizing antibodies against SARS‐CoV‐2 variants induced by natural infection or vaccination: a systematic review and individual data meta‐analysis. 2021. 10.1093/cid/ciab646 [DOI]
- 53. Focosi D, Maggi F. Neutralising antibody escape of SARS‐CoV‐2 spike protein: risk assessment for antibody‐based Covid‐19 therapeutics and vaccines. Rev Med Virol. 2021;31(6):e2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bernal JL, Andrews N, Gower C, et al. Effectiveness of Covid‐19 vaccines against the B. 1.617. 2 (delta) variant. N Engl J Med. 2021;385:585‐594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Haveri A, Ekström N, Solastie A, et al. Persistence of neutralizing antibodies a year after SARS‐CoV‐2 infection in humans. Eur J Immunol. 2021;51(12):3202‐3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Haralambieva IH, Monroe JM, Ovsyannikova IG, Grill DE, Poland GA, Kennedy RB. Homologous and variant‐specific memory B‐Cell and antibody responses after SARS‐CoV‐2 mRNA vaccination. medRxiv. 2022;jiac042. [DOI] [PMC free article] [PubMed]
- 57. Connor BA, Couto‐Rodriguez M, Barrows JE, et al. Monoclonal antibody therapy in a vaccine breakthrough SARS‐CoV‐2 hospitalized delta (B. 1.617. 2) variant case. Int J Infect Dis. 2021;110:232‐234. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the sequences generated in the current study are submitted to the GISAID that are available at “https://www.gisaid.org/login/” under the accession numbers: EPI_ISL_3462498‐ EPI_ISL_3462527, EPI_ISL_3553499‐EPI_ISL_3553528, EPI_ISL_3827529‐EPI_ISL_3827557.


