FIGURE 3.
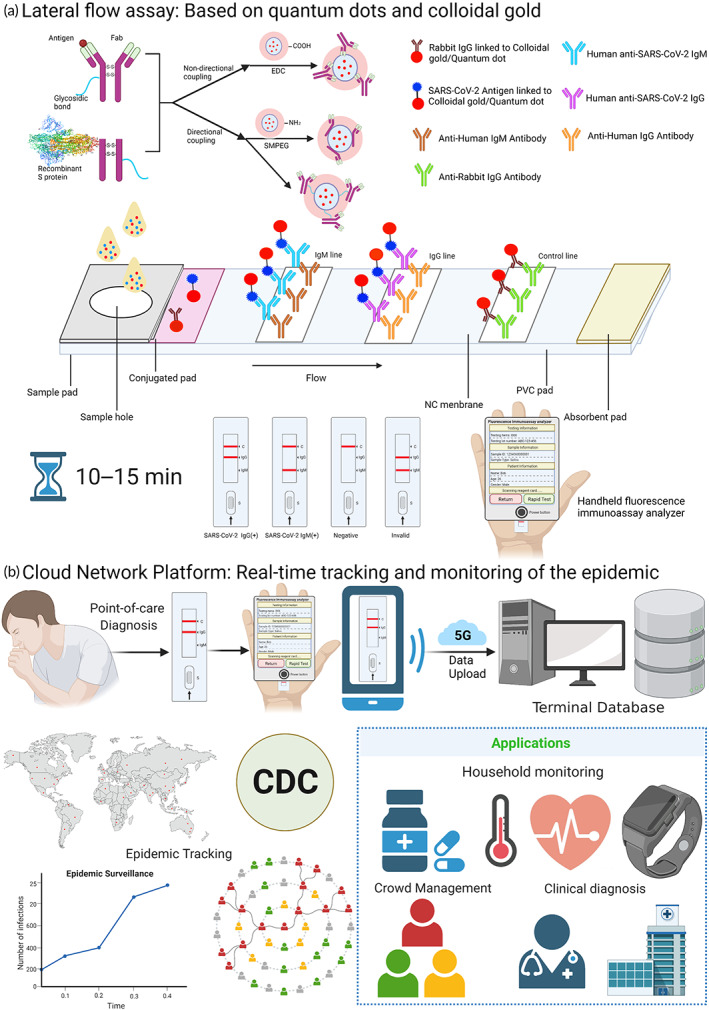
Serological detection of SARS‐CoV‐2 (a) Lateral flow assay: Quantum dots/colloidal gold can couple antibodies via specific labeling (using agent Maleamide–polyethylene glycol–succinimide ester (SMPEG)) and nonspecific labeling (using EDC/NHS chemistry methods). The rapid quantum dot and colloidal gold immunodiagnostic method for SARS‐CoV‐2 antibody‐based on high specificity recombinant protein and quantum dot/colloidal gold immunofluorescence probes by double antibody sandwich or indirect method methodology using lateral flow assay. The patient sample added to the sample pad will move to the absorbent pad along the NC membrane by chromatography, which will form the tagged‐antibody–antigen–antibody complex. After 10–15 min, test results can be observed on the test kit and operators can get an accurate fluorescence signal by a handheld fluorescent immunoanalyzer. (b) Cloud Network Platform: Rapid test kits can be used at the point of care for suspicious population screening tests, mobile devices such as cell phones can be used for result identification, handheld fluorescent immunoassay analyzers can perform a quantitative and qualitative analysis of test results, and qualitative and quantitative data can be uploaded to the terminal database, the CDC can manage relevant infections and suspicious populations through analysis of qualitative and quantitative data, give relevant clinical diagnosis recommendations, and combine with wearable devices such as smartwatches to achieve daily monitoring of people's medication, body temperature, heart rate, and other vital signs at the point of care such as communities and families, to control the development of epidemics in a timely and effective manner.
