Abstract
Rationale
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) causes long‐term pulmonary sequelae in adults, but little is known about pulmonary outcomes in pediatrics.
Objective(s)
The aim of this study was to describe long‐term subjective and objective pulmonary abnormalities after SARS‐CoV‐2 infection in pediatric populations.
Methods
Single‐center, retrospective cohort of patients seen in post‐coronavirus disease 2019 (COVID‐19) pulmonary clinic in 2021. Subjects evaluated had persistent pulmonary symptoms 4 weeks or more after initial infection. Clinical testing included a 6‐min walk test (6MWT), chest X‐ray, pre‐ and postbronchodilator spirometry, plethysmography, and diffusion capacity. Patients were followed 2‐to‐3‐months after the initial visit with repeat testing. The primary outcome was the presence of abnormal pulmonary function testing. Secondary measures included variables associated with pulmonary outcomes.
Results
Eighty‐two adolescents were seen at a median of 3.5 months postinfection, with approximately 80% reporting two or more symptoms at clinic presentation (cough, chest pain, dyspnea at rest, and exertional dyspnea). At follow‐up (~6.5 months) exertional dyspnea persisted for most (67%). Spirometry was normal in 77% of patients, but 31% had a positive bronchodilator response. No abnormalities were noted on plethysmography or diffusion capacity. Clinical phenotypes identified included inhaled corticosteroid responsiveness, paradoxical vocal fold motion disorder, deconditioning, and dysautonomia. Multivariable modeling demonstrated that obesity, anxiety, and resting dyspnea were associated with reduced 6MWT, while female sex and resting dyspnea were associated with higher Borg Dyspnea and Fatigues scores.
Conclusions
This is the largest study to date of pediatric patients with long‐term pulmonary sequelae post‐COVID‐19. Identified clinical phenotypes and risk factors warrant further study and treatment.
Keywords: COVID‐19, dyspnea, PASC
1. INTRODUCTION
Severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2) has continued to spread throughout the United States since the pandemic was declared in March of 2020. 1 The spread of the delta variant led to a large peak in US pediatric infections by early fall 2021, with pediatric cases making up to 30% of total cases depending on the prevalence of disease in a given region and even higher caseloads seen with the omicron variant in winter of 2021. 2
Long‐term postacute sequelae of SARS‐CoV‐2 infection (PASC) have been increasingly described in adults. While PASC encompasses a wide spectrum of symptoms, subjective respiratory‐specific complaints include dyspnea, cough, chest tightness, and fatigue. 3 , 4 , 5 Of the variety of lung abnormalities noted long‐term, decreased diffusing capacity is the most common lung function abnormality reported in adults. 6 , 7 , 8 , 9 Similar subjective complaints have been noted in pediatric patients even after mild or asymptomatic disease, with limited data on pulmonary function changes. 10 Recent data from a small cohort in Philadelphia corroborates that exertional dyspnea, cough, and exercise intolerance are the most common long‐term pulmonary symptoms among children with PASC. 11 However, there is sparse data on how these symptoms and objective abnormalities in pulmonary testing evolve over time.
We initiated a Pediatric Pulmonary Post‐COVID clinic in February 2021 due to an increasing referral volume of children with prolonged pulmonary symptoms after coronavirus disease 2019 (COVID‐19), including shortness of breath, difficulty exercising, and prolonged cough. Based on the paucity of data in children, a standardized clinical testing approach was applied (see methods). The aim of this study was to describe the subjective and objective pulmonary abnormalities of this patient population, identify different patterns of clinical presentation, and analyze changes in pulmonary findings over time.
2. METHODS
2.1. Study design
The study was approved by the Nationwide Children's Hospital Institutional Review Board (STUDY00001357). This was a single‐center, retrospective cohort study on all patients evaluated in the Post‐COVID Pulmonary clinic at Nationwide Children's Hospital between February 2021 and December 2021. The electronic healthcare record (EHR) was utilized for subject identification and data collection in an electronic database.
2.2. Standardized clinical evaluation
Patients were referred for evaluation of persistent pulmonary symptoms 4 weeks or more after initial SARS‐CoV‐2 infection (no chronic symptoms before infection). Patients were managed by one of three pulmonary physicians using a standardized clinical testing approach. Inclusion criteria included confirmed laboratory testing for SARVS‐CoV‐2 (n = 78) or consistent symptoms and exposure to a household contact at the time of illness (n = 4). At a minimum, all subjects were evaluated at an initial visit and recommended to follow‐up in 2–3 months. Each initial visit included a 6‐min walk test (6MWT) with Borg rating of perceived exertion revised scale, chest radiography (CXR), pre‐ and postbronchodilator spirometry, plethysmography, and diffusion capacity of lung for carbon monoxide (DLCO) testing. All subjects met with a pulmonary physician who completed a history and physical exam, subsequently ordering further clinical testing as indicated. Initial illness symptoms were self‐reported.
All objective testing was performed in accordance with American Thoracic Society standards. Spirometry was performed using Vitalgraph while plethysmography and DLCO testing were performed on Koko Px machines using Nspire software. PFT data were normalized for age, race, gender, and height using Global Lung Function Initiative (GLI) norms. Obstructive deficits were defined as a forced expiratory volume in one second (FEV1) to forced vital capacity (FVC) ratio of less than 80% predicted. Bronchodilator response was defined as an increase in FEV1 by 12% or more from baseline or an increase in forced expiratory flow (FEF) 25%–75% by 25% or more. Restrictive deficits were defined as total lung capacity (TLC) less than 80% on plethysmography. Similarly, DLCO was considered normal over 80% predicted, after correction for hemoglobin and alveolar volume. 6MWT results were evaluated based on published sex and age norms. 12 Imaging studies were interpreted by a pediatric radiologist, per standard clinical care.
2.3. Statistical analyses
Data distributions were visualized and quality checked with bar plots for categorical variables and violin plots for continuous variables. Data were summarized using frequency (percentage) for categorical variables and median (interquartile range [IQR]) for continuous variables. Outcomes evaluated at both initial and follow‐up visits included 6MWT distance, Borg Dyspnea, and Fatigue scores, and change in heart rate from before to after the 6MWT. Clinic visits were compared using a paired t test or Wilcoxon signed‐rank test, as appropriate, and visualized with violin plots.
Univariable linear regression models were fit for outcomes collected at the initial clinic visit, including 6MWT, Borg Dyspnea, Borg Fatigue, and change in heart rate during the 6MWT. Independent variables tested for association with the outcomes included demographics (age, sex, race, and insurance status), secondhand environmental exposures (vaping and tobacco), sports participation, history of comorbidities (obesity, anxiety, asthma, and hypertension), vaccination status, and symptoms at clinic presentation (chest pain, cough, dyspnea, and shortness of breath during exercise). Multivariable linear regression models were fit to model the same outcomes. To prevent over‐fitting models due to sampling size limitations, a separate multivariable model was fit for each comorbidity and presentation symptom, adjusting for age, sex, race, and insurance status. Bronchodilator response was analyzed using univariable logistic regression, but we were unable to fit a multivariable model due to sample size. Two‐sided p values <0.05 were considered statistically significant. All statistical analysis was conducted in R version 4.0 (R Core Team, Vienna, Austria) with reproducible programming in R Markdown.
3. RESULTS
3.1. Cohort demographics
The clinical characteristics of the enrolled cohort are listed in Table 1 with initial visit outcomes in Table 2 and follow‐up outcomes in Table 4. Paired follow‐up outcomes as described below are shown in E‐Table S1. Most individuals were white, competitive athletes referred after mild, and symptomatic outpatient infections confirmed by laboratory testing. Twenty‐nine percent (n = 24) had a history of asthma, with the majority (75.0%, n = 18) characterized as well‐controlled mild intermittent with no preventative inhalers or albuterol only prescribed, no albuterol use in the month before initial infection, and no previous spirometry performed in our network. Additional five subjects were classified as well‐controlled, mild persistent asthma before infection, requiring preventative inhaled corticosteroids only with no prior subspecialty care. COVID‐19 history and symptoms at the time of initial infection by patient and caregiver report are listed in Table 3, with cough and shortness of breath being the most prominent initial symptoms. Importantly, all symptoms were new after the initial SARS‐CoV‐2 infection. A median of 3.5 months had passed from initial infection until the time of clinic evaluation. Approximately 50% of the cohort had at least one follow‐up visit 2–3 months later, at a median of 6.7 months postinitial infection. Only 23% of the cohort had received at least one COVID vaccination dose at initial presentation. Five patients (6%) suffered a second confirmed SARS‐CoV‐2 infection during the study period while still undergoing pulmonary evaluation and treatment for sequelae of their first infection. Only one patient was previously hospitalized for Multisystem Inflammatory Syndrome in Children (MIS‐C) associated with COVID‐19.
Table 1.
Demographics and comorbidities
| Characteristic | N = 82 a |
|---|---|
| Demographics | |
| Age (years) | 15.2 (2.3) |
| Female sex | 48 (58.5%) |
| Race | |
| Asian | 1 (1.2%) |
| Black | 8 (9.8%) |
| Hispanic | 3 (3.7%) |
| Multiracial | 4 (4.9%) |
| White | 66 (80.5%) |
| Insurance | |
| Private | 61 (74.4%) |
| Public | 18 (22.0%) |
| Uninsured | 3 (3.7%) |
| BMI (kg/m2) | 21.8 (19.7–25.8) |
| BMI percentile | 67.6 (45.6–91.8) |
| Vaping exposure | 9 (11.0%) |
| Tobacco exposure | 13 (15.9%) |
| Competitive athlete | 66 (80.5%) |
| Pulmonary follow‐up | 40 (49.4%) |
| Seen by cardiology | 59 (72.0%) |
| Co‐morbidities | |
| Obesity | 17 (20.7%) |
| Hypertension | 4 (4.9%) |
| Anxiety | 22 (27.2%) |
| Asthma | 24 (29.3%) |
| Type of asthma | |
| Mild intermittent | 18 (75.0%) |
| Mild persistent | 5 (20.8%) |
| Moderate persistent | 1 (4.2%) |
Abbreviations: BMI, body mass index; IQR, interquartile range; SD, standard deviation.
Mean (SD); n (%); median (IQR).
Table 2.
Outcomes at initial visit.
| Characteristic | N = 82 a | Abnormal a |
|---|---|---|
| Lung function measures | ||
| Normal spirometry | 63 (76.8%) | |
| Bronchodilator response | 24 (30.8%) | |
| FVC prebronchodilator (%) | 104 (13) | 4 (4.9) |
| FVC postbronchodilator (%) | 105 (13) | 3 (3.7) |
| FVC change (%) | 0.3 (3.5) | |
| FEV1 prebronchodilator (%) | 104 (97–111) | 4 (4.9) |
| FEV1 postbronchodilator (%) | 111 (98–116) | 2 (2.4) |
| FEV1 change (%) | 5 (0–8) | |
| FEF 25%–75% prebronchodilator (%) | 96 (85–114) | 4 (4.9) |
| FEF 25%–75% postbronchodilator (%) | 112 (102–126) | 3 (3.7) |
| FEF 25%–75% change (%) | 16 (2–26) | |
| Total lung capacity (%) | 99 (12) | 5 (6.1) |
| Residual volume (%) | 19 (13–26) | 1 (1.2) |
| Diffusing capacity (%) | 118 (17) | 0 |
| Oxygen saturation (%) | 98 (97–99) | 0 |
| 6MW outcomes | ||
| 6MWD (m) | 578 (503–610) | |
| HR pre‐6MW (bpm) | 88 (15) | |
| HR post‐6MW (bpm) | 137 (22) | |
| HR 6 MW change (bpm) | 49 (22) | |
| Borg dyspnea post 6 MW | 3 (2–4) | |
| Borg fatigue post 6MW | 3 (1–5) |
Abbreviations: 6MWD, 6‐min walk distance; FEF, forced expiratory flow; FEV, forced expiratory volume; FVC, forced vital capacity; HR, heart rate; IQR, interquartile range; SD, standard deviation.
n (%); mean (SD); median (IQR).
Table 4.
COVID follow‐up history, symptoms, and outcomes
| Characteristic | N = 40 a |
|---|---|
| Follow‐up COVID history | |
| Vaccinated | 15 (37.5%) |
| Pulmonary rehab referral | 5 (13.2%) |
| Follow‐up symptoms | |
| Months post‐COVID | 6.7 (4.9, 8.6) |
| Dyspnea | 6 (15.0%) |
| Cough | 5 (12.5%) |
| Chest pain | 8 (20.0%) |
| SOB during exercise | 27 (67.5%) |
| Follow‐up bronchodilator measures | |
| Normal spirometry | 26 (89.7%) |
| FVC prebronchodilator (%) | 100 (12) |
| FEV1 prebronchodilator (%) | 101 (92–106) |
| FEF 25%–75% prebronchodilator (%) | 90 (80–107) |
| Oxygen saturation (%) | 98.0 (97.0–98.5) |
| Follow‐up 6MW outcomes | |
| 6 MWD (m) | 594 (549–614) |
| HR pre‐6MW (bpm) | 84 (19) |
| HR post‐6MW (bpm) | 131 (25) |
| HR 6 MW change (bpm) | 47 (13) |
| Borg dyspnea post 6 MW | 3.0 (2.0–4.0) |
| Borg fatigue post 6MW | 3.0 (1.5–5.0) |
Abbreviations: 6MWD, 6‐min walk distance; FEF, forced expiratory flow; FEV, forced expiratory volume; FVC, forced vital capacity; HR, heart rate; IQR, interquartile range; SD, standard deviation.
n (%); mean (SD); median (IQR).
Table 3.
COVID‐19 history, symptoms at infection, and symptoms at clinic presentation
| Characteristic | N = 82 a |
|---|---|
| COVID history | |
| Re‐infected | 5 (6.1%) |
| Vaccinated | 19 (23.2%) |
| Hospitalized | 7 (8.5%) |
| MIS‐C | 1 (1.2%) |
| Pulmonary rehab referral | 7 (8.6%) |
| Symptoms at infection | |
| Fever | 40 (50.0%) |
| Cough | 58 (70.7%) |
| Chest pain | 34 (41.5%) |
| Shortness of breath | 51 (62.2%) |
| Upper respiratory infection | 46 (58.2%) |
| Sore throat | 31 (37.8%) |
| Headache | 43 (52.4%) |
| Malaise at infection | 25 (31.2%) |
| Myalgia | 38 (46.3%) |
| GI symptoms | 21 (25.9%) |
| Anosmia | 39 (47.6%) |
| Ageusia | 32 (39.0%) |
| Presyncope | 15 (18.5%) |
| Palpitations | 19 (23.5%) |
| Symptoms at clinic presentation | |
| Months post‐COVID | 3.5 (1.9–5.8) |
| Chest pain | 50 (61.0%) |
| Cough | 25 (30.5%) |
| Dyspnea | 42 (51.2%) |
| SOB during exercise | 74 (90.2%) |
Abbreviations: GI, gastrointestinal; IQR, interquartile range; MIS‐C, multisystem inflammatory syndrome in children; SOB, shortness of breath.
n (%); median (IQR).
3.2. Functional limitations
Pulmonary symptoms at clinic presentation (Table 3) included cough (31%), chest pain (61%), dyspnea at rest (51%), and exertional dyspnea (90%). Most patients (~80%) presented with two or more symptoms. At follow‐up, exertional dyspnea improved but remained common (68%), with other symptoms significantly improved (cough 12%, chest pain 20%, and dyspnea at rest 15%). 6MWTs were performed in all patients at presentation. Normalized reference values for pediatric 6MWTs are variable in the literature, but as a reference, 610 m is equivalent to 2000 feet, or a little over 1/3 of a mile walked. The median distance walked in 6 min is presented in Figure 1A (median [IQR] 577.6 [503.2–609.6 m]). Twenty‐seven individuals had an available 6MWT at follow‐up, with a significant difference in overall median distance walked found between paired visits (Figure 1B, p = 0.039, follow‐up 6MWT 594.4 [549.1–614.2] m). Sixty‐seven percent of these individuals had increased walk distances from the first visit, including the six lowest initial distances (Figure 1B). Minimal clinically important differences for change in 6‐min walk test distance of adults have been reported from 14–30 m, but these have not been reported in pediatric populations. 13
Figure 1.
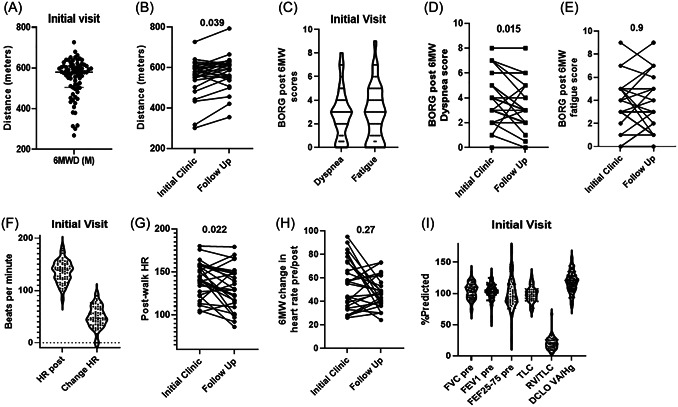
Abnormalities in pulmonary testing. (A) Median distance (meters) walked during 6 min for the entire cohort during initial (n = 82) pulmonary clinic evaluation. Initial values median 577.6 (IQR 503.2–609.6) [range 268–727]. (B) Paired values for individuals with an available initial and follow‐up visit 6MWT. p‐value = 0.039, n = 27. Follow‐up values median 594.4 (IQR 549.1–614.2) [range 355–792]. (C) Initial visit Borg dyspnea (median 3.0, IQR 2.0–4.0) and fatigue (median 3.0, IQR 1.0–5.0) scores post‐6MWT, n = 82. (D) Paired values for Borg dyspnea scores post‐6MWT for individuals with initial and follow‐up (median 3.0, IQR 2.0–4.0) scores. p‐value = 0.015, n = 27. (E) Paired values for Borg fatigues scores post‐6MWT for individuals with initial and follow‐up (median 3.0, IQR 1.5–5.0) scores. p‐value > 0.9, n = 27. (F) Post‐walk heart rate (HR post) and change in heart rate (change HR) values during 6MWT at initial visits, n = 82. Paired values for postwalk (G) and change in heart rate (H) during 6MWT at initial and follow‐up visits. Post heart‐rate p‐value = 0.022, change in heart rate p‐value = 0.27, n = 27. (I) Percent predicted spirometry (FVC, FEV1, and FEF25%–75%), plethysmography (TLC, RV/TLC), and diffusing capacity (DLCO) values for all participants at initial evaluation, n = 82. All p‐values were calculated using the Wilcoxon signed‐rank test. 6MWD, 6‐min walk distance; FEF, forced expiratory flow; FEV, forced expiratory volume; FVC, forced vital capacity; HR, heart rate; IQR, interquartile range
Borg dyspnea and fatigue scores post 6MWT were elevated in most of the cohort at initial visit, equivalent to moderate perceived symptoms (Figure 1C, median dyspnea 3.0, median fatigue 3.0). At follow‐up, perceived dyspnea post‐walk was significantly improved (p = 0.015, Figure 1D) but fatigue remained unchanged (p = 0.9, Figure 1E). In addition to increased fatigue and dyspnea postwalk, heart rate variability during the walk was observed at both initial (Figure 1F) and follow‐up (Figure 1GH) visits. Significant tachycardia was observed (median postwalk heart rate 140 beats per minute) and median increase in heart rate was 47 beats pre‐ and postwalk (change). At follow‐up, postwalk heart rates decreased for most individuals (median post‐walk heart rate 129 bpm, p = 0.022, Figure 1G), while change in heart rate was unaffected with heart rates increasing a median of 47 beats per minute postwalk (p value 0.5, Figure 1H). It is noted that the seven individuals with the most variable heart rates at initial visits all showed significant improvement at follow‐up (Figure 1H).
3.3. Changes in pulmonary function testing
Pulse oximetry measurements ranged from 96% to 100% in all participants. Lung function and volume measurements for the cohort at presentation are presented in Table 2 and Figure 1I. Overall, spirometry measurements were within normal limits in 77% of the cohort, with approximately 15% (n = 14) presenting with obstructive deficits and five patients with restrictive deficits. Prior lung function measurements were not available for the cohort. Additionally, 31% of the cohort had a positive bronchodilator response. Thirty‐eight percent of the individuals with a bronchodilator response had a prior history of asthma (9/24). Secondhand smoke or vape exposure was not associated with increased bronchodilator responses, with only 4/22 exposed individuals (18%) with a positive response. No major abnormalities were observed in plethysmography or diffusing capacity measurements. Due to normal baseline spirometry measurements in most individuals, only 29/40 patients agreed to spirometry at a follow‐up visit. Ninety percent of follow‐up spirometry studies were normal, therefore further analyses in this sub‐group were not performed (data in E‐Table S1).
3.4. Diagnostic phenotypes
Based on clinical history, family history, and bronchodilator testing, 43% of individuals were prescribed an inhaled corticosteroid or inhaled corticosteroid/long‐acting beta agonist combination. Only two individuals with a prior history of asthma had normal spirometry and bronchodilator testing and did not require steroid treatment. Eighty‐five percent of treated individuals reported a clinical response to prescribed medications at follow‐up, including improved pulmonary function testing. Based on clinical history and normal functional testing, 13% of individuals were diagnosed with paradoxical vocal fold motion disorder (PVFMD) in conjunction with ENT laryngoscopy and were treated with speech therapy. Flow‐volume loop abnormalities were not observed in this sub‐group. An additional 13% enrolled in pulmonary rehab due to persisting functional limitations. Evaluations for diagnosis and treatment of dysautonomia (based on symptom report and suggested by 6MWT) are currently pending for a further subset of individuals.
3.5. Risk factors associated with clinical outcomes
We performed univariable and multivariable modeling to determine risk factors associated with alterations in 6MWT, Borg scoring, heart rate responses at initial clinic visits, and bronchodilator responsiveness (E‐Table S2–S6). In univariable models, obesity, anxiety, cough, and resting dyspnea were associated with decreased 6MWT distance while competitive athletes were associated with increased distance. Multivariable models showed similar associations with obesity, anxiety, cough, and dyspnea at presentation remaining significant, however, dyspnea during exercise was also associated with decreased 6MWT after adjusting for age, sex, race, and insurance status. Female sex and dyspnea during exercise were associated with higher Borg Dyspnea scores in univariable models, with female sex and dyspnea at rest and during exercise significant in the multivariable models. Similarly, female sex and resting and exertional dyspnea were associated with elevated Borg Fatigue scores in univariable and multivariable models. There were no significant factors associated with heart rate alterations during 6MWT or bronchodilator responses during spirometry. A summary of multivariable linear regression models is found in E‐Table S6.
4. DISCUSSION
To our knowledge, this study represents the largest study in the United States evaluating long‐term pulmonary PASC in pediatric patients and includes follow‐up data to allow analysis of changes in pulmonary findings over time. Despite a mostly mild, outpatient SARS‐CoV‐2 infection, patients in this cohort experienced significant, persistent respiratory symptoms leading to clinical evaluation. Our work identifies at least three clinical phenotypes that warrant further exploration. These include patients with airway hyperreactivity, PVFMD, and those with subjective dyspnea/fatigue and few objective abnormalities. A fourth phenotype of dysautonomia that has been described in adults 14 is undergoing current study.
Our study population's objective response to bronchodilators and subjective improvement with asthma therapies highlights the importance of exploring bronchodilator responsiveness in pediatric patients reporting persistent dyspnea, even if they don't have a personal or family history of asthma. Leftin Dobkin et al. 11 also identified a substantial proportion of pediatric patients with positive bronchodilator response in their patient cohort (28.6% of total subjects), though post‐bronchodilator spirometry was not uniformly performed and thus may have been underestimated. A large prospective, cohort study of pediatric patients hospitalized in Russia identified the presence of allergic disease (including asthma, allergic rhinitis, eczema, or food allergy) as a predictor for persistent symptoms following SARS‐CoV‐2 infection. 15 A recent large US pediatric study also demonstrated worsened asthma control in the first six months after SARS‐CoV‐2 infection. 16 Adult studies evaluating long‐term PASC have not looked at bronchodilator responsiveness, with most focusing on abnormal DLCO correlation with acute disease severity. 6 , 9 , 17 The underlying cause of worsening small airway obstruction is unclear, though work is being done to better define how SARS‐CoV‐2 impacts patients with underlying asthma, with recognition that its clinical effect varies between patients with type 2 (allergic, eosinophilic) and nontype 2 asthma. 18 A lack of overall changes in lung function in our pediatric cohort compared to existing adult data may reflect changing immunologic responses with age or differences in airway reparative response.
For those patients in our cohort who were diagnosed with PVFMD in conjunction with Otolaryngology, laryngeal control therapy was recommended with guidance by speech therapy. The true prevalence of PVFMD in the general population is unknown, though it is a well‐established cause of persistent chest tightness and dyspnea. 19 Higher rates of PVFMD are seen in patients with asthma than compared with control subjects, 20 and co‐morbid diagnoses of anxiety or anxiety‐related disorders are often present and can complicate treatment responses. 21 The SARS‐CoV‐2 pandemic has been associated with increased anxiety and psychologic co‐morbidities in teenagers as seen in our cohort. 22 One patient in our cohort was also diagnosed with laryngeal sensory neuropathy, successfully treated with superior laryngeal nerve block. Peripheral neuropathies are a known result of SARS‐CoV‐2 infection, 23 with case reports of unilateral recurrent laryngeal nerve palsy and vocal cord paralysis reported in adults. 24 , 25 Specific pathophysiologic mechanisms underlying increased PVFMD post‐SARS‐CoV‐2 remain undefined. Therefore, improved screening and diagnosis of PVFMD post‐SARS‐CoV‐2 along with long‐term follow‐up on treatment results are important areas for future research. Additionally, our findings would suggest that integration of behavioral health specialists into pediatric PASC clinics may be warranted, as personal report of anxiety was also associated with reduced distance on 6MWT. Formal anxiety screening measures were not in place at the time of initial evaluations, therefore overall anxiety prevalence may be underestimated.
Virtually all patients presenting to the clinic experienced notable fatigue and dyspnea, often at rest and worse with exertion. Roughly half of these subjects had no significant abnormalities on chest imaging, lung function testing, or physical exam. Many of these subjects were high‐functioning athletes involved in school and club sports. While we did not use a standardized measure of the quality of life (QOL) as part of their clinical care, each patient noted the significant impact these symptoms had on their daily life at school, during sports, and at home. Dyspnea and fatigue are the most common long‐term PASC symptoms in adults, with 44% of patients noting the worsened quality of life with these symptoms. 26 Several immunopathological mechanisms have been discussed as potential etiologies for these persistent PASC symptoms, most involving abnormal cellular or humoral immune responses. 27 However, detailed mechanistic studies in the pediatric population with pulmonary‐PASC symptoms have not been performed. It is unclear why we observed an association between female sex and increased dyspnea and fatigue scores, but increased symptoms in large cohorts of adult female survivors have also been described. 28
Across each of the above diagnostic categories, subjects consistently demonstrated significant tachycardia and heart rate variability during 6MWT, with most walking distances below the normalized medians for age, sex, and height. At follow‐up visits, 6MWT distance and tachycardia improved, while dyspnea scores decreased. Heart rate variability and fatigue scores remained statistically unchanged. Adult patients followed after hospitalization for severe SARS‐CoV‐2 have shorter 6MWT distances, 29 higher resting heart rates, and lower peak oxygen uptake during cardiopulmonary exercise testing compared with controls at hospital follow‐up. 29 , 30 Limited data exists on 6MWT changes over time in the pediatric population. The heart rate variability seen in our population raises the possibility of autonomic dysfunction, and tachycardia during 6MWT was also reported in six adolescents with exercise intolerance in Leftin Dobkin's study. 31 SARS‐CoV‐2 is known to cause immune‐mediated neurological syndromes, 23 , 32 and autonomic dysregulation may result from significant viral infection. 33 While we had not included evaluation for orthostatic intolerance syndromes in our initial clinical approach to these patients, the notable heart rate variability present suggests the need for expanded evaluation and treatment of these syndromes moving forward.
The study population presented is limited to patients referred to the Pediatric Pulmonary Post‐COVID clinic, and therefore does not likely represent the entire PASC experience of pediatric patients or those with pre‐existing chronic lung diseases. Patients were predominantly white, privately insured, competitive athletes, highlighting disparities in initial care access for non‐White populations. This is in contrast to the demographic distribution of reported SARS‐CoV‐2 cases in Ohio, with only 61% White patients. 2 Because of this difference, subsequent to this study we performed community outreach, engaged care coordinators who follow at‐risk children with public insurance, and educated community and hospital providers on referral disparities to allow improved access for underrepresented minority and under‐resourced patients experiencing PASC pulmonary symptoms. These engagements have also increased referrals for non‐athletes.
All patients were symptomatic, though most had not been hospitalized and only one had MIS‐C, suggesting mild acute initial COVID infections. Re‐infections could also bias results. Our results contrast with others reporting long‐term outcomes following mild or asymptomatic COVID disease. Researchers in Australia studied 171 children after SARS‐CoV‐2 infection causing mild or asymptomatic disease. 10 The majority of these patients were fully recovered within weeks of symptom onset, and respiratory symptoms were mild, though patients were younger than our study population (median age 3 years, IQR 1–8). While this study describes PASC findings in adolescents with mild COVID disease, our population likely does not adequately reflect the experience of patients who had been hospitalized with severe COVID. We expect to see more children post‐hospitalization moving forward as Pediatric hospitalization rates continue to rise.
Patients who were too young to complete pulmonary function testing were not evaluated as a part of this clinic and many individuals declined lung function testing at follow‐up if initial testing was normal or symptoms had improved. Additionally, the retrospective nature of this study limits our ability to better understand the reason many patients failed to return for follow‐up. It is unclear whether these patients had complete resolution of their respiratory symptoms or encountered barriers to follow‐up care. Unfortunately, this poor follow‐up rate may result in bias involving data from return visits.
Despite the limitations above, this study provides the largest report on pediatric patients with PASC symptoms to date. We highlight at least three clinical phenotypes to help guide further diagnosis and treatment of pediatric patients with dyspnea and fatigue following mild SARS‐CoV‐2 infection. Additionally, we determined risk factors associated with worsened long‐term clinical outcomes. Prospective studies evaluating other diagnostic methods (lung clearance index, cardiopulmonary exercise testing, orthostatic instability evaluation, etc.) and pathophysiology are critical to impact the significant morbidity experienced by pediatric patients with PASC. Additionally, efforts should be made to better understand correlations between sociodemographic and environmental exposures, immunologic measures, and structural or functional lung abnormalities following SARS‐CoV‐2 infection. Continued evaluation of the frequency of PASC symptoms in emerging SARS‐CoV‐2 variants remains of interest.
AUTHOR CONTRIBUTIONS
Benjamin T. Kopp, Katelyn Krivchenia, and Sabrina Palacios developed the clinic and performed clinical assessments. Katelyn Krivchenia, Sabrina Palacios, Mariah Eisner, Simon Lee, and Benjamin T. Kopp contributed to study design. Benjamin T. Kopp, Bailey Young, and Simon Lee maintained the database. Benjamin T. Kopp and Mariah Eisner performed statistical analyses. Sabrina Palacios, Katelyn Krivchenia, Mariah Eisner, Octavio Ramilo, Asuncion Mejias, and Benjamin T. Kopp wrote the manuscript. All authors edited the manuscript.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Supporting information
Supplementary information.
ACKNOWLEDGMENTS
Thank you to our respiratory therapists for their performance of the clinical testing used in this manuscript. No funding support was required for this manuscript.
Palacios S, Krivchenia K, Eisner M, et al. Long‐term pulmonary sequelae in adolescents post‐SARS‐CoV‐2 infection. Pediatric Pulmonology. 2022;57:2455‐2463. 10.1002/ppul.26059
Sabrina Palacios and Katelyn Krivchenia contributed equally to the study.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Dong E, Du H, Gardner L. An interactive web‐based dashboard to track COVID‐19 in real time. Lancet Infect Dis. 2020;20(5):533‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Center for Disease Control and Prevention . COVID Data Tracker. 2021. https://covid.cdc.gov/covid-data-tracker/#demographicsovertime
- 3. Nalbandian A, Sehgal K, Gupta A, et al. Post‐acute COVID‐19 syndrome. Nat Med. 2021;27(4):601‐615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Greenhalgh T, Knight M, A'Court C, Buxton M, Husain L. Management of post‐acute covid‐19 in primary care. BMJ. 2020;370:m3026. [DOI] [PubMed] [Google Scholar]
- 5. Shah W, Hillman T, Playford ED, Hishmeh L. Managing the long term effects of covid‐19: summary of NICE, SIGN, and RCGP rapid guideline. BMJ. 2021;372:n136. [DOI] [PubMed] [Google Scholar]
- 6. Blanco JR, Cobos‐Ceballos MJ, Navarro F, et al. Pulmonary long‐term consequences of COVID‐19 infections after hospital discharge. Clin Microbiol Infect. 2021;27(6):892‐896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. de Graaf MA, Antoni ML, Ter Kuile MM, et al. Short‐term outpatient follow‐up of COVID‐19 patients: a multidisciplinary approach. EClinicalMedicine. 2021;32:100731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wu Q, Zhong L, Li H, et al. A follow‐up study of lung function and chest computed tomography at 6 months after discharge in patients with coronavirus disease 2019. Can Respir J. 2021;2021:6692409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bellan M, Soddu D, Balbo PE, et al. Respiratory and psychophysical sequelae among patients with COVID‐19 four months after hospital discharge. JAMA Netw Open. 2021;4(1):e2036142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Say D, Crawford N, McNab S, Wurzel D, Steer A, Tosif S. Post‐acute COVID‐19 outcomes in children with mild and asymptomatic disease. Lancet Child Adolesc Health. 2021;5(6):e22‐e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Leftin Dobkin SC, Collaco JM, McGrath‐Morrow SA. Protracted respiratory findings in children post‐SARS‐CoV‐2 infection. Pediatr Pulmonol. 2021;56:3682‐3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Geiger R, Strasak A, Treml B, et al. Six‐minute walk test in children and adolescents. J Pediatr. 2007;150(4):395‐399.e2. [DOI] [PubMed] [Google Scholar]
- 13. Bohannon RW, Crouch R. Minimal clinically important difference for change in 6‐minute walk test distance of adults with pathology: a systematic review. J Eval Clin Pract. 2017;23(2):377‐381. [DOI] [PubMed] [Google Scholar]
- 14. Buoite Stella A, Furlanis G, Frezza NA, Valentinotti R, Ajcevic M, Manganotti P. Autonomic dysfunction in post‐COVID patients with and witfhout neurological symptoms: a prospective multidomain observational study. J Neurol. 2021;269:587‐596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Osmanov IM, Spiridonova E, Bobkova P, et al. Risk factors for long covid in previously hospitalised children using the ISARIC global follow‐up protocol: a prospective cohort study. Eur Respir J . 2021. [DOI] [PMC free article] [PubMed]
- 16. Chou CC, Morphew T, Ehwerhemuepha L, Galant SP. COVID‐19 infection may trigger poor asthma control in children. J Allergy Clin Immunol Pract . 2022. [DOI] [PMC free article] [PubMed]
- 17. Mo X, Jian W, Su Z, et al. Abnormal pulmonary function in COVID‐19 patients at time of hospital discharge. Eur Respir J . 2020;55(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Skevaki C, Karsonova A, Karaulov A, Xie M, Renz H. Asthma‐associated risk for COVID‐19 development. J Allergy Clin Immunol. 2020;146(6):1295‐1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Morris MJ, Christopher KL. Diagnostic criteria for the classification of vocal cord dysfunction. Chest. 2010;138(5):1213‐1223. [DOI] [PubMed] [Google Scholar]
- 20. Yelken K, Yilmaz A, Guven M, Eyibilen A, Aladag I. Paradoxical vocal fold motion dysfunction in asthma patients. Respirology. 2009;14(5):729‐733. [DOI] [PubMed] [Google Scholar]
- 21. Gavin LA, Wamboldt M, Brugman S, Roesler TA, Wamboldt F. Psychological and family characteristics of adolescents with vocal cord dysfunction. J Asthma. 1998;35(5):409‐417. [DOI] [PubMed] [Google Scholar]
- 22. Walsh K, Furey WJ, Malhi N. Narrative review: COVID‐19 and pediatric anxiety. J Psychiatr Res. 2021;144:421‐426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ftiha F, Shalom M, Jradeh H. Neurological symptoms due to coronavirus disease 2019. Neurol Int. 2020;12(1):8639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tin S, Foo F, Breitling M, Saverimuttu J, Lisi C. A new and rare presentation of unilateral recurrent laryngeal nerve palsy in a COVID‐19 patient with no recent history of endotracheal intubation. Cureus. 2021;13(9):e17700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Korkmaz MO, Guven M. Unilateral vocal cord paralysis case related to COVID‐19. SN Compr Clin Med. 2021;3(11):1‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Carfi A, Bernabei R, Landi F. Persistent symptoms in patients after acute COVID‐19. JAMA Network. 2020;324(6):603‐605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ramakrishnan RK, Kashour T, Hamid Q, Halwani R, Tleyjeh IM. Unraveling the mystery surrounding post‐acute sequelae of COVID‐19. Front Immunol. 2021;12:686029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Huang C, Huang L, Wang Y, et al. 6‐month consequences of COVID‐19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220‐232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Raman B, Cassar MP, Tunnicliffe EM, et al. Medium‐term effects of SARS‐CoV‐2 infection on multiple vital organs, exercise capacity, cognition, quality of life and mental health, post‐hospital discharge. EClinicalMedicine. 2021;31:100683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Daher A, Balfanz P, Cornelissen C, et al. Follow up of patients with severe coronavirus disease 2019 (COVID‐19): pulmonary and extrapulmonary disease sequelae. Respir Med. 2020;174:106197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Leftin Dobkin SC, Collaco JM, McGrath‐Morrow SA. Protracted respiratory findings in children post‐SARS‐CoV‐2 infection. Pediatr Pulmonol. 2021;56(12):3682‐3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Guilmot A, Maldonado Slootjes S, Sellimi A, et al. Immune‐mediated neurological syndromes in SARS‐CoV‐2‐infected patients. J Neurol. 2021;268(3):751‐757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Dani M, Dirksen A, Taraborrelli P, et al. Autonomic dysfunction in ‘long COVID’: rationale, physiology and management strategies. Clin Med (Lond). 2021;21(1):e63‐e67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


