Abstract
Objective
Data on COVID‐19 outcomes in persons with epilepsy (PWE) are scarce and inconclusive. We aimed to study the risk of hospitalization and death for COVID‐19 in a large cohort of PWE from March 1, 2020 to October 31, 2021.
Methods
The historical cohort design (EpiLink Bologna) compared adult PWE grouped into people with focal epilepsy (PFE), idiopathic generalized epilepsy (PIGE), and developmental and/or epileptic encephalopathy (PDEE), and a population cohort matched (ratio 1:10) for age, sex, residence, and comorbidity (assessed with the multisource comorbidity score), living in the local health trust of Bologna (approximately 800 000 residents). Clinical data were linked to health administrative data.
Results
In both cohorts (EpiLink: n = 1575 subjects, 1128 PFE, 267 PIGE, 148 PDEE, 32 other; controls: n = 15 326 subjects), 52% were females, and the mean age was 50 years (SD = 18). Hospital admissions for COVID‐19 in the whole period were 49 (3.1%) in PWE and 225 (1.5%) in controls. The adjusted hazard ratio (aHR) in PWE was 1.9 (95% confidence interval [CI] = 1.4–2.7). The subgroups at higher risk were PFE (aHR = 1.9, 95% CI = 1.3–2.8) and PDEE (aHR = 3.9, 95% CI = 1.7–8.7), whereas PIGE had a risk comparable to the controls (aHR = 1.1, 95% CI = .3–3.5). Stratified analyses of the two main epidemic waves (March–May 2020, October 2020–May 2021) disclosed a higher risk of COVID‐19‐related hospitalization during the first epidemic wave (March–May 2020; aHR = 3.8, 95% CI = 2.2–6.7). Polytherapy with antiseizure medications contributed to a higher risk of hospital admission. Thirty‐day risk of death after hospitalization was 14% in both PWE and controls.
Significance
During the first 20 months since the outbreak of COVID‐19 in Bologna, PWE had a doubled risk of COVID‐19 hospital admission compared to a matched control population. Conversely, epilepsy did not represent a risk factor for COVID‐19‐related death.
Keywords: antiseizure medication (ASM), epidemiology, epileptic encephalopathy, mortality, outcome
Key Points.
During the first 20 months of the pandemic in Bologna, PWE had a doubled risk of COVID‐19 hospital admission compared to controls
The subgroups at higher risk were persons with focal epilepsy and epileptic encephalopathy
There was a higher risk of COVID‐19‐related hospitalization in the first epidemic wave (March–May 2020)
Polytherapy with antiseizure medications contributed to a higher risk of hospital admission
Epilepsy did not represent a risk factor for COVID‐19‐related death
1. INTRODUCTION
Coronavirus disease 2019 (COVID‐19) is an ongoing pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS‐CoV‐2), which emerged in Wuhan, China, in early December 2019, and rapidly spread worldwide. Italy was the first country in the western world to have been heavily affected by COVID‐19, with the first case recorded on February 20, 2020. Despite several adopted measures, including the implementation of mass vaccination campaigns since early 2021, at the time of writing COVID‐19 still represents the major public health burden, in Italy as in the world. Emilia‐Romagna, an administrative region in northern Italy whose capital city is Bologna, has been hard hit since the early COVID‐19 outbreak.
The risk of severe COVID‐19 and related case fatality is higher among patients with chronic disorders, including dementia and cerebrovascular, cardiovascular, respiratory, and renal conditions. 1 , 2 Epilepsy is a chronic neurological condition accounting for a significant proportion of the world's disease burden. 3 Persons with epilepsy (PWE) show a paradigmatic pattern of vulnerability, due to seizures, antiseizure medication (ASM)‐related adverse events, and, in patients with developmental and/or epileptic encephalopathies (DEE), intellectual disability. A meta‐analysis of studies published up to June 2021 showed that epilepsy is associated with poor COVID‐19 outcomes. 4 However, these data are mostly derived from population studies referring to the first year of the pandemic in which PWE were not clinically characterized and were represented in a limited number.
This historical cohort study aimed to assess the risk of hospitalization and death for COVID‐19 in a large cohort of PWE referred to a tertiary epilepsy center, compared with a matched control population. The period of observation was 20 months from the epidemic onset. Stratified analyses of the two main epidemic waves (March–May 2020, October 2020–May 2021) were also performed.
2. MATERIALS AND METHODS
2.1. Design and standard protocol approvals
This was a historical cohort study. A similar approach has been previously used to study COVID‐19 outcomes in persons with Parkinson disease or parkinsonism during the first epidemic wave (March–May 2020) in the local health trust of Bologna (LHTB). 5 The STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) 6 and RECORD (Reporting of Studies Conducted Using Observational Routinely‐Collected Health Data) 7 guidelines were followed. This study was approved by the local institutional review board (reference number: 574‐2021‐OSS‐AUSLBO).
2.2. Setting and study population
The LHTB covers almost all the metropolitan area of Bologna, located in northern Italy, and had a population of 752 104 adults on December 31, 2019. At the beginning of the outbreak, the area included nine general hospitals, three academic hospitals (one general, one neurologic, one orthopedic), and 11 private clinics operating within the Italian National Health Service, for a total of approximately 4400 beds, of which approximately 120 were in intensive care units (ICUs). In response to the COVID‐19 outbreak, hospital facilities underwent a major reorganization; in the early outbreak, eight COVID‐19 hospitals/wards were created, with a total of approximately 550 dedicated beds, of which approximately 150 were in ICUs. Thereafter, multiple further logistical reorganizations were related to the current epidemiological situation. During the study period, two different epidemic waves can be considered: the first wave from March 2020 to May 2020 and the second wave from October 2020 to May 2021 (https://www.salute.gov.it/portale/nuovocoronavirus).
Patients were recruited at the Epilepsy Center of the Institute of Neurological Sciences of Bologna, Scientific Institute for Research and Health Care, which follows the vast majority of PWE in the LHTB, ranging from persons referred for a suspected first seizure or a new diagnosis of epilepsy, to persons with rare and complex epilepsies. All adult patients living in the LHTB who underwent a visit during 2018 and 2019 and were alive on March 1, 2020, were screened by two authors (L.M., L.T.), and those with a diagnosis of epilepsy based on International League Against Epilepsy criteria 8 were included in the study (EpiLink cohort). Subsequently, patients were classified into four categories: patients with idiopathic generalized epilepsy (PIGE), patients with focal epilepsy (PFE), patients with DEE (PDEE), and patients with a different or unknown epilepsy type (PUE). 9
The control cohort consisted of people matched with the EpiLink cohort, with a ratio of 1:10 for age, sex, district of residence, and comorbidity according to the Multisource Comorbidity Score (MCS), a novel comorbidity score based on data from administrative health sources that was validated in a large Italian cohort, including the Emilia‐Romagna region. 10
2.3. Data sources
The Italian health care system is universal; therefore, all resident citizens are anonymously recorded in a homogenous way when accessing any public or private health facility, and personal data are stored by the qualified local health trust in secure databases. The Epidemiology and Statistics Unit of the Institute of Neurological Sciences of Bologna, Scientific Institute for Research and Health Care (part of the LHTB) has access to several health administrative databases (outpatient tests and visits, emergency department admissions, hospital discharge, drug prescription, mortality). The health data used by this study have almost 100% coverage, as the recording is mandatory at any access. Each individual of the two cohorts (EpiLink cohort, control cohort) was linked with administrative databases.
Information from the drug prescription database was used to further classify the included PWE into two groups: polytherapy (PWE‐P; i.e., those who were regularly prescribed ≥2 ASMs) and nonpolytherapy (PWE‐NP). The MCS was based on data from the hospital discharge and drug prescription databases.
2.4. Outcomes
The hospital admission rate for COVID‐19 from March 1, 2020 to October 31, 2021 was the primary outcome. Diagnosis was obtained from hospital discharge data. Hospital admissions for COVID‐19 were captured using an algorithm of the Emilia‐Romagna region based on the International Classification of Diseases, Ninth Revision, Clinical Modification codes (October 28, 2020; https://www.ospfe.it/il‐professionista/ufficio‐dimissioni‐ospedaliere‐info‐e‐strumenti/allegati‐2/normativa/20210201NotaREReGU26.pdf). We also assessed the 30‐day mortality risk of death for any cause, calculated in the group of hospitalized people.
2.5. Statistical analysis
In the descriptive analysis, the characteristics of the two cohorts are presented as mean and SD for the continuous variables and with absolute (n) and relative (%) frequency for categorical variables. Chi‐squared test and Kruskal–Wallis test were used to evaluate the univariate association between conditions (epilepsy/controls) and categorical or continuous variables, respectively. To evaluate the outcomes (hospitalization for COVID‐19 and death), two analyses were performed.
In the first one, the time to entry in the analysis was March 1, 2020, and the time to endpoint was the date of hospital admission, death, or October 31, 2021, whichever came first. We used univariate and multivariate Cox regression models to estimate the hazard ratio (HR) with a 95% confidence interval (CI) associating COVID‐19 hospitalization to the presence of epilepsy. Results of the univariate analysis are presented using Kaplan–Meier curves. In the multivariate models, we included sex, age, MCS, district of residence, and the number of hospital admissions in 2018 and 2019 (propensity to hospitalization) as covariates. The proportional hazards assumption was tested (p > .05) using Schoenfeld residuals. We performed different types of subgroup analyses. In the first one, we evaluated the hospital admission rate in the different diagnostic categories: PFE, PIGE, and PDEE. In the second one, we divided each category of diagnosis into polytherapy (persons who were regularly prescribed ≥2 ASMs) and nonpolytherapy (<2 ASMs): PWE‐P and PWE‐NP; PFE‐P and PFE‐NP; PDEE‐P and PDEE‐NP; PIGE‐P and PIGE‐NP. Finally, separate stratified analyses were performed for the first and second epidemic peak for each diagnostic group: March–May 2020 and October 2020–May 2021.
For the second outcome, the overall 30‐day mortality risk in the group of hospitalized people, the time to entry was the date of hospital admission. The results were presented as absolute (n) and relative (%) frequency and the differences between groups as evaluated by chi‐squared test.
Data linkage and statistical analyses were conducted using Stata SE version 14.2.
3. RESULTS
The study population inclusion flowchart is shown in Figure S1. The EpiLink cohort included 1575 PWE (mean age = 50 ± 18 years, 52% females), the majority of whom were PFE (72%), followed by PIGE (17%), PDEE (9%), and PUE (2%). More than one third of the patients were prescribed ≥2 ASMs (PWE‐P), with higher percentages among PDEE (Table 1). The control cohort included 15 326 subjects with the same demographic features (Table S1).
TABLE 1.
Features of the EpiLink cohort, subdivided as persons with focal epilepsy, epileptic encephalopathy, and idiopathic generalized epilepsy, on March 1, 2020
| Feature | Patients with: | p a | Total persons with epilepsy b | ||
|---|---|---|---|---|---|
| Focal epilepsy | Epileptic encephalopathy | Idiopathic generalized epilepsy | |||
| n | 1128 | 148 | 267 | 1575 | |
| Mean age, years (SD) | 53.9 (17.5) | 38.6 (15.5) | 41.1 (16.8) | <.001 | 50.2 (18.2) |
| Age distribution, n (%) | <.001 | ||||
| 18–29 years | 120 (10.6) | 58 (39.2) | 85 (31.8) | 267 (17.0) | |
| 30–39 years | 127 (11.3) | 19 (12.8) | 56 (21.0) | 207 (13.1) | |
| 40–49 years | 204 (18.1) | 28 (18.9) | 44 (16.5) | 285 (18.1) | |
| 50–59 years | 229 (20.3) | 28 (18.9) | 43 (16.1) | 304 (19.3) | |
| 60–69 years | 204 (18.1) | 12 (8.1) | 20 (7.5) | 240 (15.2) | |
| 70–79 years | 159 (14.1) | 3 (2.0) | 15 (5.6) | 183 (11.6) | |
| 80–89 years | 81 (7.2) | 0 (0) | 4 (1.5) | 85 (5.4) | |
| ≥90 years | 4 (.4) | 0 (0) | 0 (0) | 4 (.3) | |
| Sex, n (%) | .197 | ||||
| Male | 546 (48.4) | 77 (52.0) | 116 (43.5) | 757 (48.1) | |
| Female | 582 (51.6) | 71 (48.0) | 151 (56.5) | 818 (51.9) | |
| District, n (%) | .031 | ||||
| Bologna | 491 (43.5) | 73 (49.3) | 127 (47.5) | 704 (44.7) | |
| Reno | 143 (12.7) | 22 (14.9) | 30 (11.2) | 200 (12.7) | |
| Pianura Est | 225 (20.0) | 30 (20.3) | 42 (15.7) | 306 (19.4) | |
| Pianura Ovest | 114 (10.1) | 7 (4.7) | 28 (10.5) | 150 (9.5) | |
| Appennino | 49 (4.3) | 6 (4.1) | 23 (8.6) | 80 (5.1) | |
| San Lazzaro | 106 (9.4) | 10 (6.8) | 17 (6.4) | 135 (8.6) | |
| Multisource comorbidity score, n (%) | <.001 | ||||
| 0–4 | 842 (74.7) | 87 (58.8) | 225 (84.3) | 1175 (74.6) | |
| 5–9 | 172 (15.3) | 41 (27.7) | 27 (10.1) | 246 (15.6) | |
| 10–14 | 58 (5.1) | 10 (6.8) | 8 (3.0) | 79 (5.0) | |
| 15–19 | 26 (2.3) | 6 (4.0) | 5 (1.9) | 39 (2.5) | |
| ≥20 | 30 (2.6) | 4 (2.7) | 2 (.7) | 36 (2.3) | |
| Number of hospital admissions in 2018 and 2019, n (%) | .064 | ||||
| 0 | 784 (69.5) | 103 (69.6) | 205 (76.8) | 1116 (70.9) | |
| 1 | 188 (16.7) | 18 (12.2) | 39 (14.6) | 250 (15.9) | |
| 2 | 83 (7.4) | 12 (8.1) | 12 (4.5) | 108 (6.9) | |
| 3 | 73 (6.5) | 15 (10.1) | 11 (4.1) | 101 (6.4) | |
| Number of therapies, n (%) | <.001 | ||||
| 0 | 89 (7.9) | 12 (8.1) | 21 (7.9) | 125 (7.9) | |
| 1 | 633 (56.1) | 49 (33.1) | 171 (64.0) | 872 (55.4) | |
| 2 | 284 (25.2) | 47 (31.8) | 55 (20.6) | 395 (25.1) | |
| ≥3 | 122 (10.8) | 40 (27.0) | 20 (7.5) | 183 (11.6) | |
Probability value shows the difference in features between the three groups of patients (focal epilepsy, developmental and/or epileptic encephalopathy, idiopathic generalized epilepsy).
The total includes 32 patients with “other type” of epilepsy.
The course of hospital admissions for COVID‐19 in the EpiLink cohort and controls is shown in Figure 1. Hospital admissions for COVID‐19 in the whole study period were 49 (3.1%) in PWE and 225 (1.5%) in controls (adjusted HR [aHR] = 1.9, 95% CI = 1.4–2.7). The subgroups at higher risk were PFE (aHR = 1.9, 95% CI = 1.3–2.8), and PDEE (aHR = 3.9, 95% CI = 1.7–8.7), whereas PIGE had a risk comparable to the controls (Table 2). ASM polytherapy contributed to a higher risk of COVID‐19‐related hospitalization, which was driven by PFE‐P (Table 2 , Figure 2).
FIGURE 1.
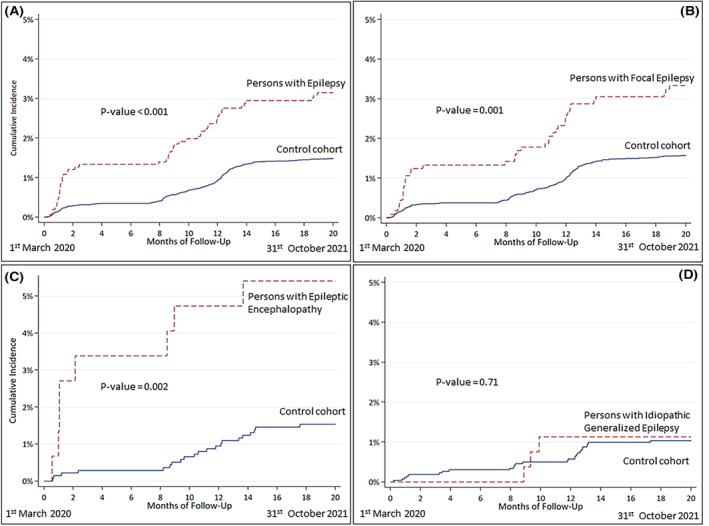
Course of hospital admissions for COVID‐19 in the EpiLink cohort and controls.
TABLE 2.
Risk of hospital admission for COVID‐19 from March 1, 2020 to October 31, 2021 in the three diagnostic groups and in the total cohort (PFE, PDEE, PIGE, and total PWE): Multivariate Cox regression models (controls as the reference group)
| Comparison | Patients with: | Total persons with epilepsy, aHR (95% CI) | ||
|---|---|---|---|---|
| Focal epilepsy, aHR (95% CI) | Epileptic encephalopathy, aHR (95% CI) | Idiopathic generalized epilepsy, aHR (95% CI) | ||
| Epilepsy vs. controls | 1.9 (1.3–2.8) | 3.9 (1.7–8.7) | 1.0 (.3–3.3) | 1.9 (1.4–2.7) |
| Polytherapy vs. controls | 2.8 (1.7–4.6) | 2.8 (.9–8.3) | 1.1 (.3–4.6) | 2.7 (1.7–4.1) |
| Nonpolytherapy vs. controls | 1.5 (.9–2.4) | 6.2 (2.0–19.9) | .9 (.1–6.7) | 1.5 (.99–2.4) |
| Age in years | 1.04 (1.03–1.05) | 1.07 (1.04–1.10) | 1.04 (1.01–1.06) | 1.04 (1.03–1.05) |
| Sex | ||||
| Male | Ref. | Ref. | Ref. | Ref. |
| Female | .5 (.4–.7) | .7 (.3–1.6) | 1.1 (.5–2.3) | .6 (.5–.8) |
| District | ||||
| Bologna | Ref. | Ref. | Ref. | Ref. |
| Reno | 1.0 (.7–1.6) | .8 (.2–2.8) | .6 (.1–2.7) | .9 (.6–1.4) |
| Pianura Est | 1.0 (.7–1.4) | .8 (.2–3.1) | .8 (.2–2.7) | .8 (.6–1.2) |
| Pianura Ovest | 1.0 (.6–1.6) | 3.3 (.7–15.6) | 1.9 (.6–5.8) | 1.1 (.7–1.6) |
| Appennino | .7 (.3–1.6) | .8 (.2–3.1) | 1.1 (.2–4.9) | .6 (.3–1.2) |
| San Lazzaro | 1.2 (.8–1.9) | 1.4 (.3–6.5) | 1.2 (.3–4.3) | 1.1 (.8–1.7) |
| Multisource comorbidity score | ||||
| 0–4 | Ref. | Ref. | Ref. | Ref. |
| 5–9 | 1.9 (1.4–2.6) | 1.5 (.6–3.8) | .9 (.1–7.7) | 1.9 (1.4–2.6) |
| 10–14 | 2.0 (1.3–3.1) | 2.8 (.8–9.5) | 3.5 (1.3–9.0) | 2.0 (1.3–3.1) |
| ≥15 | 3.9 (2.4–6.2) | 1.5 (.3–6.9) | 4.2 (.9–18.6) | 3.9 (2.4–6.2) |
| Number of hospital admissions in 2018 and 2019 | ||||
| 0 | Ref. | Ref. | Ref. | Ref. |
| 1 | .9 (.6–1.3) | 2.1 (.8–5.3) | 1.1 (.4–3.2) | .9 (.6–1.3) |
| 2 | 1.5 (.9–2.3) | 2.2 (.8–5.5) | 1.1 (.2–4.9) | 1.5 (.9–2.3) |
| 3 | 2.4 (1.6–3.5) | 3.4 (.99–11.5) | 2.2 (.6–7.4) | 2.4 (1.6–3.5) |
Note: In a subgroup analysis, we evaluated patients with and without polytherapy in two different groups (controls as the reference group).
Abbreviations: aHR, adjusted hazard ratio; CI, confidence interval; PDEE, patients with developmental and/or epileptic encephalopathy; PFE, patients with focal epilepsy; PIGE, patients with idiopathic generalized epilepsy; PWE, persons with epilepsy; Ref., reference.
Values are written in bold when the 95% confidence interval does not cross the line of no difference (=1).
FIGURE 2.
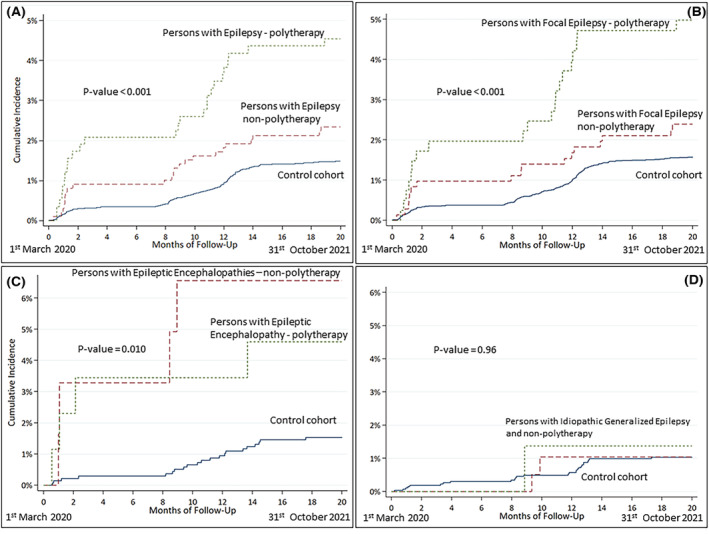
Course of hospital admissions for COVID‐19 in the EpiLink cohort stratified by antiseizure polytherapy and controls.
The risk of hospitalization for COVID‐19 in PWE compared to controls was significantly higher in the first epidemic wave (aHR = 3.9, 95% CI = 2.2–6.7), where it was driven by PFE and PDEE (both P and NP), whereas during the second wave the risk was higher only in PWE‐P compared to controls (aHR = 1.9, 95% CI = 1.1–3.5) and was driven by PFE‐P (aHR = 2.3, 95% CI = 1.9–3.5; Table 3, Figure 3).
TABLE 3.
Risk of hospital admission for COVID‐19 in the two different epidemic periods (the first from March 1, 2020 to May 31, 2020, and the second from October 1, 2020 to May 31, 2021) in the three diagnostic groups and in the total cohort (PFE, PDEE, PIGE, and total PWE): Multivariate Cox regression models with control group as reference
| Comparison | Patients with: | Total persons with epilepsy, aHR a (95% CI) | ||
|---|---|---|---|---|
| Focal epilepsy, aHR a (95% CI) | Epileptic encephalopathy, aHR a (95% CI) | Idiopathic generalized epilepsy, aHR a (95% CI) | ||
| March 1, 2020 to May 31, 2020 | ||||
| Epilepsy vs. controls | 3.3 (1.7–6.4) | 10.9 (2.8–43.0) | Without cases | 3.9 (2.2–6.7) |
| Polytherapy vs. controls | 4.5 (1.9–10.3) | 11.5 (2.5–53.3) | ‐ | 5.5 (2.8–10.9) |
| Nonpolytherapy vs. controls | 2.7 (1.2–6.1) | 10.3 (1.6–64.0) | ‐ | 2.9 (1.4–5.9) |
| October 1, 2020 to May 31, 2021 | ||||
| Epilepsy vs. controls | 1.5 (.93–2.5) | 1.7 (.5–6.1) | 1.5 (.4–5.1) | 1.5 (.96–2.3) |
| Polytherapy vs. controls | 2.3 (1.2–4.3) | .8 (.1–6.2) | 1.3 (.2–10.1) | 1.9 (1.1–3.5) |
| Nonpolytherapy vs. controls | 1.1 (.6–2.2) | 4.2 (.9–20.5) | 1.6 (.4–7.1) | 1.2 (.7–2.1) |
Note: In a subgroup analysis, we evaluated patients with and without polytherapy in two different groups.
Abbreviations: aHR, adjusted hazard ratio; CI, confidence interval; PDEE, patients with developmental and/or epileptic encephalopathy; PFE, patients with focal epilepsy; PIGE, patients with idiopathic generalized epilepsy; PWE, persons with epilepsy.
Values are written in bold when the 95% confidence interval does not cross the line of no difference (=1).
Adjusted for age, sex, district of residence, multisource comorbidity score, and number of hospital admissions in 2018 and 2019.
FIGURE 3.
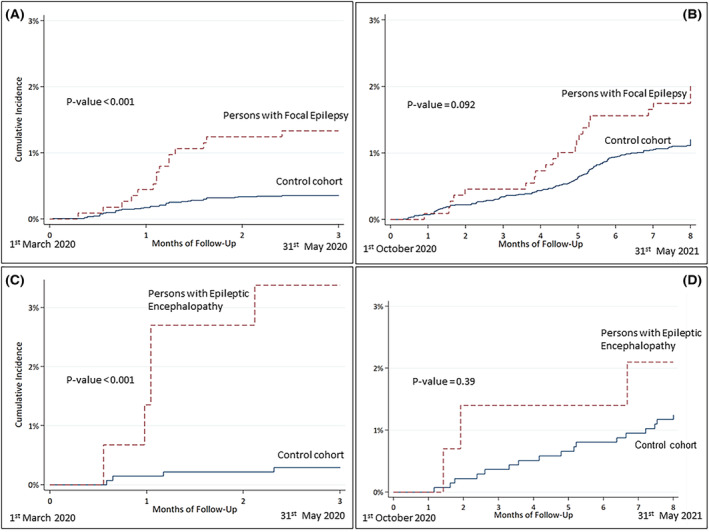
Course of hospital admissions for COVID‐19 in the EpiLink cohort (persons with focal epilepsy and persons with epileptic encephalopathy) and controls stratified into the two epidemic waves (March–May 2020, October 2020–May 2021).
Detailed clinical documentation regarding hospitalization was available for 36 of 49 PWE (25 PFE, eight PDEE, three PIGE). Among these, in 15 PWE (42%) epilepsy‐related factors played a determinant role in the decision to admit the patient or were the reason for presentation to the emergency department: four due to seizures; three due to ASM management/adverse events; and eight due to the complexity of the underlying disease, including epilepsy (four of whom were infected in a long‐stay residential care home, 3 PDEE, 1 PFE).
The 30‐day risk of death after hospitalization was 14% in both PWE and controls (p = .93), with no statistically significant difference based on epilepsy type, although a trend toward a higher risk was found in DEE compared with matched controls (25% vs. 14%, p = .49). Mortality was not influenced by either ASM polytherapy or epidemic wave.
4. DISCUSSION
During the first 20 months since the COVID‐19 outbreak in the local health trust of Bologna, PWE had an approximately twofold risk of hospitalization for COVID‐19 compared with a matched general population cohort, which was driven by PFE, the largest group, and PDEE, the group at higher risk. Conversely, the risk of death after hospitalization was the same in PWE and controls, suggesting that epilepsy did not influence the COVID‐19 fatality rate in our cohort.
Compared to previous reports studying the impact of COVID‐19 on PWE, our study had several strongpoints. First, PWE were selected among patients referred to a high‐volume tertiary epilepsy center, thus allowing a reliable epilepsy diagnosis based on international criteria and an accurate classification into epilepsy types. The EpiLink cohort was representative of the entire spectrum of epilepsies, with percentages of PFE, PIGE, and PDEE comparable to those reported in the literature. 11 We were also able to further subclassify PWE based on the prescription of ASM: approximately one third was on ASM polytherapy, a fraction consistent with that of drug‐resistant epilepsy (DRE) among adult PWE. 12 Additionally, the reason for hospital admission was assessed on an individual basis for PWE. All this information was not provided in any previous study with the same aim, 4 , 13 , 14 , 15 yet it is paramount to correctly interpret the two main outcomes: risk of hospitalization and death for COVID‐19 in PWE.
The control group was matched using the MCS, a novel comorbidity score showing a better performance than both the Charlson and Elixhauser scores. 10 PWE and controls were all living in the LHTB and were matched for the district of residence, thus allowing homogenization of the influence that geographical context and capacity of the health care system may have on COVID‐19 incidence and mortality in the two groups. 16
A higher hospitalization risk for COVID‐19 in PWE was previously described; 13 , 14 specifically, a large British population study reported an approximate aHR in PWE of 1.75 (95% CI = 1.52–2.02), similar to ours. 14 The reasons underlying this finding, however, have not been specifically evaluated. In the EpiLink cohort, we verified that almost half of the PWE with COVID‐19‐related hospitalization were admitted, in addition to SARS‐CoV‐2 infection, for epilepsy‐related reasons, including seizure clusters, ASM adverse events, or the complexity of the underlying epilepsy syndrome and/or of ASM polytherapy. In the multivariate analysis, the HR was adjusted, in addition to the matching variables, by hospitalizations for any cause that occurred in 2018 and 2019, thus making COVID‐19 itself arguably responsible for the increased hospitalization risk in PWE compared with matched controls. Several epilepsy‐related factors might be influenced by COVID‐19. First, although the risk of seizure worsening for most PWE who develop COVID‐19 is low, 17 , 18 acute symptomatic seizures may occur during COVID‐19, as in other febrile infections. 19 , 20 Some studies have reported risk factors for seizure exacerbation during the pandemic, regardless of SARS‐CoV‐2 infection, including epilepsy severity and ASM polytherapy, sleep disorders, social factors, and mental stress in general. 21 , 22 , 23 , 24 Notably, in a survey‐based study performed at our epilepsy center between May and July 2020, a clinical worsening was reported in 27 of 222 PWE (12%) and was associated with limited access to health care, among other factors. 23 Epilepsy care was negatively impacted during the pandemic for several reasons, including restricted access to out‐ and inpatient clinics and routine electroencephalograms, in addition to difficulties related to ASM prescription and testing of blood levels. 17 , 25 Moreover, emergency department operators might have had an increased propensity to hospitalize PWE with COVID‐19, due to their vulnerability.
A further strongpoint of our study was to assess the risk of hospitalization for COVID‐19 during a 20‐month‐long period, thus including two relatively well‐defined epidemic waves (March–May 2020 and October 2020–May 2021). We found that the hospitalization risk in PWE compared with controls was remarkably high only during the first wave, whereas during the second wave this was slightly increased only for PFE‐P and not for PWE in general. An explanation for this difference may be that, during the first wave, the abovementioned psychological, social, and health care‐related issues were more prominent, thus facilitating seizures and epilepsy‐related admissions. Accordingly, in a recently published Korean study, PWE underwent a transient seizure exacerbation in 2020, which was followed by a trend toward seizure amelioration in 2021. 24 Additionally, the propensity of emergency department operators to hospitalize PWE with COVID‐19 might have been more pronounced during the first epidemic wave, when the clinical features of COVID‐19 and its impact on epilepsy were largely unknown. 17 Finally, PWE and other vulnerable populations were prioritized to receive SARS‐CoV‐2 vaccination starting in January 2021, and this may have contributed to lowering the hospitalization risk during the second wave.
PDEE were the population at highest risk of hospitalization, likely because among PWE they represent the most vulnerable patients, are more frequently institutionalized, and might not be compliant with the use of personal protective equipment, resulting in a higher risk of infection. 26 , 27 , 28 On the other hand, PIGE had the same risk as controls, possibly due to the lower disease burden of this subgroup compared with the other included epilepsy types. 29 Similarly, the higher risk of hospitalization for COVID‐19 that we detected in PWE‐P is likely attributable to the corresponding increased epilepsy severity, namely DRE, and to ASM‐related adverse events, as there is no evidence that taking ASM increases per se the risk of infection or complications.
In our study, both PWE and controls hospitalized for COVID‐19 had a 14% 30‐day risk of death, in line with the fatality rate reported in the literature for hospitalized COVID‐19 patients during the same period. 30 Contrasting with our findings, some previous studies reported a higher risk of death from COVID‐19 in PWE, 4 , 13 , 14 and epilepsy has been included by the US Centers for Disease Control and Prevention on a list of conditions that may increase the risk of serious COVID‐19 infection, likely because it is a chronic neurological condition.
It is possible that the relatively small number of events (i.e., deaths) in the included PWE precluded this observation. However, it is also possible that the mortality risk potentially attributable to certain epilepsy syndromes has been mitigated by the accurate matching of PWE with controls having the same burden of comorbidities, assessed with the MCS. Accordingly, a recent study in which PWE were matched with propensity score matching did not find an increased susceptibility to COVID‐19‐related mortality among PWE compared with controls. 15 Further large‐scale population studies are needed to address this question.
4.1. Limitations
Only adult patients were included in our study; COVID‐19 outcomes in pediatric PWE might differ and should be investigated with studies specifically addressing this population.
Moreover, data on some potential confounders were not available, including socioeconomic status and residence in long‐stay care homes. Finally, the MCS was a proxy of the actual comorbidity status and did not include all pathologies and disease‐related conditions, including further possible confounders such as intellectual disability.
5. CONCLUSIONS
During the first 20 months since the outbreak of COVID‐19 in Bologna, Italy, adult PWE had a doubled risk of COVID‐19 hospital admission compared to a matched control population, mostly attributable to epilepsy‐related factors during the first epidemic wave. Conversely, epilepsy did not represent a risk factor for COVID‐19‐related death.
AUTHOR CONTRIBUTIONS
L.M. and C.Z. wrote the first draft. L.V. and F.Bi. supervised the study. L.M., L.T., L.L., B.M., L.D.V., E.P., L.V., P.R., L.F., F.Bi., R.M., and P.T. had a substantial role in the acquisition of clinical data. C.Z., F.Ba., and L.V. were responsible for data linkage and statistical analyses. All authors contributed to the interpretation of data, revised the manuscript for intellectual content, and approved the final version.
CONFLICT OF INTEREST
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Supporting information
APPENDIX S1
ACKNOWLEDGMENTS
We would like to thank Cristian Ceresano and Stefania Gamberini for their help in facilitating the collection of data. Open Access Funding provided by Universita degli Studi di Bologna within the CRUI‐CARE Agreement.
Muccioli L, Zenesini C, Taruffi L, Licchetta L, Mostacci B, Di Vito L, Risk of hospitalization and death for COVID‐19 in persons with epilepsy over a 20‐month period: The EpiLink Bologna cohort, Italy. Epilepsia. 2022;00:1–11. 10.1111/epi.17356
Lorenzo Muccioli and Corrado Zenesini contributed equally. Luca Vignatelli and Francesca Bisulli contributed equally.
REFERENCES
- 1. Hariyanto TI, Putri C, Arisa J, Situmeang RFV, Kurniawan A. Dementia and outcomes from coronavirus disease 2019 (COVID‐19) pneumonia: a systematic review and meta‐analysis. Arch Gerontol Geriatr. 2021;93:104299. 10.1016/j.archger.2020.104299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Geng J, Yu X, Bao H, Feng Z, Yuan X, Zhang J, et al. Chronic diseases as a predictor for severity and mortality of COVID‐19: a systematic review with cumulative meta‐analysis. Front Med (Lausanne). 2021;8:588013. 10.3389/fmed.2021.588013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. GBD 2016 Neurology Collaborators . Global, regional, and national burden of neurological disorders, 1990‐2016: a systematic analysis for the global burden of disease study 2016. Lancet Neurol. 2019;18(5):459–80. 10.1016/S1474-4422(18)30499-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Siahaan YMT, Ketaren RJ, Hartoyo V, Hariyanto TI. Epilepsy and the risk of severe coronavirus disease 2019 outcomes: a systematic review, meta‐analysis, and meta‐regression. Epilepsy Behav. 2021;125:108437. 10.1016/j.yebeh.2021.108437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vignatelli L, Zenesini C, Belotti LMB, Baldin E, Bonavina G, Calandra‐Buonaura G, et al. Risk of hospitalization and death for COVID‐19 in people with Parkinson's disease or parkinsonism. Mov Disord. 2021;36(1):1–10. 10.1002/mds.28408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. J Clin Epidemiol. 2008;61(4):344–9. 10.1016/j.jclinepi.2007.11.008 [DOI] [PubMed] [Google Scholar]
- 7. Benchimol EI, Smeeth L, Guttmann A, Harron K, Moher D, Petersen I, et al. The REporting of studies Conducted using Observational Routinely‐collected health Data (RECORD) statement. PLoS Med. 2015;12(10):e1001885. 10.1371/journal.pmed.1001885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, Elger CE, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014;55(4):475–82. 10.1111/epi.12550 [DOI] [PubMed] [Google Scholar]
- 9. Scheffer IE, Berkovic S, Capovilla G, Connolly MB, French J, Guilhoto L, et al. ILAE classification of the epilepsies: position paper of the ILAE commission for classification and terminology. Epilepsia. 2017;58(4):512–21. 10.1111/epi.13709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Corrao G, Rea F, Di Martino M, De Palma R, Scondotto S, Fusco D, et al. Developing and validating a novel multisource comorbidity score from administrative data: a large population‐based cohort study from Italy. BMJ Open. 2017;7(12):e019503. 10.1136/bmjopen-2017-019503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jallon P, Latour P. Epidemiology of idiopathic generalized epilepsies. Epilepsia. 2005;46((Suppl 9)):10–4. 10.1111/j.1528-1167.2005.00309.x [DOI] [PubMed] [Google Scholar]
- 12. Tang F, Hartz AMS, Bauer B. Drug‐resistant epilepsy: multiple hypotheses, few answers. Front Neurol. 2017;8:301. 10.3389/fneur.2017.00301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cabezudo‐García P, Ciano‐Petersen NL, Mena‐Vázquez N, Pons‐Pons G, Castro‐Sánchez MV, Serrano‐Castro PJ. Incidence and case fatality rate of COVID‐19 in patients with active epilepsy. Neurology. 2020;95(10):e1417–25. 10.1212/WNL.0000000000010033 [DOI] [PubMed] [Google Scholar]
- 14. Clift AK, Coupland CAC, Keogh RH, Diaz‐Ordaz K, Williamson E, Harrison EM, et al. Living risk prediction algorithm (QCOVID) for risk of hospital admission and mortality from coronavirus 19 in adults: national derivation and validation cohort study. BMJ. 2020;371:m3731. 10.1136/bmj.m3731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yoo J, Kim JH, Jeon J, Kim J, Song TJ. Risk of COVID‐19 infection and of severe complications among people with epilepsy: a nationwide cohort study. Neurology. 2022;98:e1886–92. 10.1212/WNL.0000000000200195 [DOI] [PubMed] [Google Scholar]
- 16. CDC COVID‐19 Response Team . Geographic differences in COVID‐19 cases, deaths, and incidence—United States, February 12–April 7, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(15):465–71. 10.15585/mmwr.mm6915e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Granata T, Bisulli F, Arzimanoglou A, Rocamora R. Did the COVID‐19 pandemic silence the needs of people with epilepsy? Epileptic Disord. 2020;22(4):439–42. 10.1684/epd.2020.1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bosak M, Mazurkiewicz I, Wężyk K, Słowik A, Turaj W. COVID‐19 among patients with epilepsy: risk factors and course of the disease. Epilepsy Behav. 2021;120:107996. 10.1016/j.yebeh.2021.107996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hwang ST, Ballout AA, Mirza U, Sonti AN, Husain A, Kirsch C, et al. Acute seizures occurring in association with SARS‐CoV‐2. Front Neurol. 2020;11:576329. 10.3389/fneur.2020.576329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Santos de Lima F, Issa N, Seibert K, Davis J, Wlodarski R, Klein S, et al. Epileptiform activity and seizures in patients with COVID‐19. J Neurol Neurosurg Psychiatry. 2021;92(5):565–6. 10.1136/jnnp-2020-324337 [DOI] [PubMed] [Google Scholar]
- 21. Assenza G, Lanzone J, Brigo F, Coppola A, di Gennaro G, di Lazzaro V, et al. Epilepsy care in the time of COVID‐19 pandemic in Italy: risk factors for seizure worsening. Front Neurol. 2020;11:737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huang S, Wu C, Jia Y, Li G, Zhu Z, Lu K, et al. COVID‐19 outbreak: the impact of stress on seizures in patients with epilepsy. Epilepsia. 2020;61(9):1884–93. 10.1111/epi.16635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mostacci B, Licchetta L, Cacciavillani C, di Vito L, Ferri L, Menghi V, et al. The impact of the COVID‐19 pandemic on people with epilepsy. An Italian survey and a global perspective. Front Neurol. 2020;11:613719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Neshige S, Aoki S, Takebayashi Y, Shishido T, Yamazaki Y, Iida K, et al. A longitudinal seizure outcome following the COVID‐19 pandemic in 2020 and 2021: transient exacerbation or sustainable mitigation. J Neurol Sci. 2021;434:120100. 10.1016/j.jns.2021.120100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cross JH, Kwon CS, Asadi‐Pooya AA, Balagura G, Gómez‐Iglesias P, Guekht A, et al. Epilepsy care during the COVID‐19 pandemic. Epilepsia. 2021;62(10):2322–32. 10.1111/epi.17045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bidoli E, Toffolutti F, Del Zotto S, Serraino D. Risk factors for territorial spreading of SARS‐CoV‐2 in North‐eastern Italy. Sci Rep. 2022;12(1):2214. 10.1038/s41598-022-05368-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Liu L, Ni SY, Yan W, Lu QD, Zhao YM, Xu YY, et al. Mental and neurological disorders and risk of COVID‐19 susceptibility, illness severity and mortality: a systematic review, meta‐analysis and call for action. EClinicalMedicine. 2021;40:101111. 10.1016/j.eclinm.2021.101111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Arain A, Shihabuddin B, Niaz F, Modur P, Taylor H, Fakhoury T, et al. Epilepsy and the impact of an epileptology clinic for patients with mental retardation and associated disabilities in an institutional setting. Epilepsia. 2006;47(12):2052–7. 10.1111/j.1528-1167.2006.00862.x [DOI] [PubMed] [Google Scholar]
- 29. Gesche J, Christensen J, Hjalgrim H, Rubboli G, Beier CP. Epidemiology and outcome of idiopathic generalized epilepsy in adults. Eur J Neurol. 2020;27(4):676–84. 10.1111/ene.14142 [DOI] [PubMed] [Google Scholar]
- 30. Macedo A, Gonçalves N, Febra C. COVID‐19 fatality rates in hospitalized patients: systematic review and meta‐analysis. Ann Epidemiol. 2021;57:14–21. 10.1016/j.annepidem.2021.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
APPENDIX S1


