Figure 2.
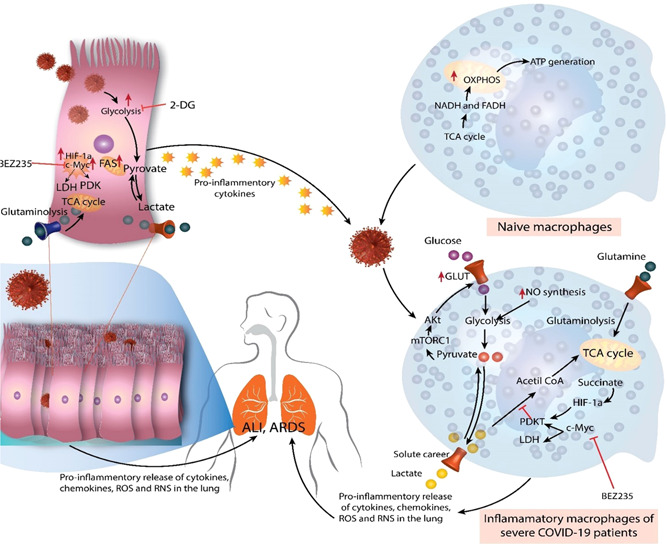
Immunometabolic reactions during coronavirus disease (COVID‐19). During homeostasis, naive macrophages (M0) do not demand substantial energy and rely primarily on oxidative phosphorylation (OXPHOS) and tricarboxylic acid (TCA) or the Krebs cycle for ATP production. Nevertheless, under the effect of the virus and pro‐inflammatory mediators generated by respiratory or pulmonary epithelial cells throughout severe acute respiratory syndrome coronavirus 2 disease, they exhibit a metabolic change. Therefore, these macrophages switch to glycolysis for fuel, which delivers faster energy than OXPHOS. The enhanced glucose absorption by these inflammatory macrophages is caused by excessive glucose transporter 1 (GLUT‐1) production, which is activated by mTORC1 signaling and causes Akt to boost GLUT1 expression. Hypoxia‐inducible factor 1‐alpha (HIF‐1a) and C‐Myc levels also increase, enhancing glycolysis by promoting lactate dehydrogenase (which converts pyruvate to lactate) and PDK1. The deposition of succinate, a TCA cycle byproduct, raises HIF‐1a concentrations. The elevated glutaminolysis contributes to the higher energy consumption of inflammatory macrophages. As a result, augmented cytokine, chemokine, reactive oxygen species (ROS), and reactive nitrogen species (RNS) production by inflammatory macrophages in the lungs leads to the "cytokine storm" that causes acute lung injury (ALI)/acute respiratory distress syndrome (ARDS). Similar to pro‐inflammatory macrophages, respiratory or alveolar epithelial cells infected with SARS‐CoV2 exhibit enhanced glycolysis, glutaminolysis, HIF‐1a, and c‐Myc overexpression. This increases the secretion of pro‐inflammatory mediators, which contributes to the "cytokine storm" and neutrophil and monocyte recruitment in the lungs of severe COVID‐19 individuals. These cells of innate immunity and immunometabolic remodeling processes cause ALI/ARDS in patients with severe COVID‐19. 30
