Evaluation of oxazolidines 2a,b and CuBr-1a,b in AAA of cinnamyl phosphate S1.
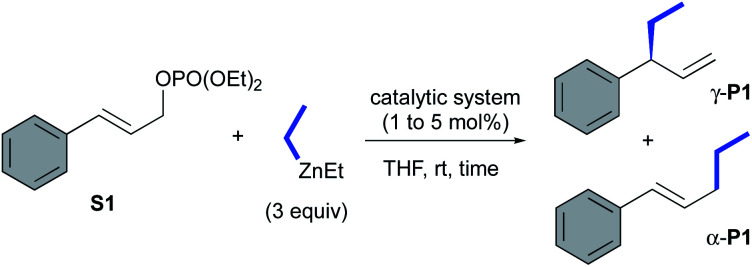
| |||||
|---|---|---|---|---|---|
| Entry | Catalytic system (mol%) | Time (h) | Conv.a (yield)b (%) | γ/α-P1 ratioc | eed (%) |
| 1e | L1aH·PF6/(CuOTf)2·tol (1.2/1) | 0.5 | >99 (62) | >99 : 1 | 90 |
| 2 | 2a/(CuOTf)2·tol (1.2/1) | 0.5 | >99 (92) | >99 : 1 | 89 |
| 3 | 2a/CuBr·SMe2 (1.2/1) | 0.5 | >99 (97) | >99 : 1 | 88 |
| 4 | CuBr-1a (1) | 0.5 | >99 (99) | >99 : 1 | 89 |
| 5 | 2a (5) | 12 | >99 (98) | >99 : 1 | 90 |
| 6 | 2a (1) | 23 | 87 (76)f | 97 : 3 | 91 |
| 7 | CuBr-1b (1) | 0.5 | >99 (88) | >99 : 1 | 87 |
| 8 | 2b (5) | 12 | Nr | Nd | Nd |
| 9 | CuBr-1a (1) with EtMgBr | 0.5 | >99 (92) | >99 : 1 | 90 |
| 10 | 2a (5) with EtMgBr | 0.5 | >99 (Nd) | 21 : 79 | 39 |
Conversions were monitored by TLC.
Isolated yields of γ-P1 after silica gel chromatography.
Molar ratio of γ/α adduct were monitored by 1H NMR spectroscopy analysis of the crude mixture.
Enantiomeric excesses were determined by chiral-phase GC analysis.
Reaction conditions: L1aH·PF6 (1.2 mol%), n-BuLi (2.5 mol%), (CuOTf)2·tol (0.5 mol%), THF, 0 °C, Et2Zn (3 equiv.), then S1, rt.
Conversion and yield were determined by 1H NMR spectroscopy (see ESI for details). Nr = no reaction. Nd = not determined.
