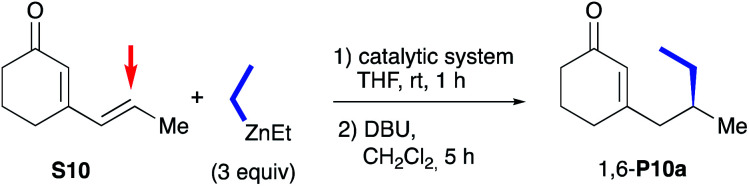
Evaluation of oxazolidines 2a,b and CuBr-1a,b in 1,6-ACA of cyclic dienones.
| ||||
|---|---|---|---|---|
| Entry | Catalytic system (mol%) | Conv.a (yield)b (%) | Selectivityc 1,6 : 1,4 | eed (%) |
| 1e | L1bH·PF6/Cu(OTf)2 (3/2) | Mr | Nd | Nd |
| 2 | 2b/Cu(OTf)2 (3/2) | >99 (Nd)f | >99 : 1 | 53 |
| 3 | 2b/CuBr·SMe2 (3/2) | >99 (Nd)f | >99 : 1 | 74 |
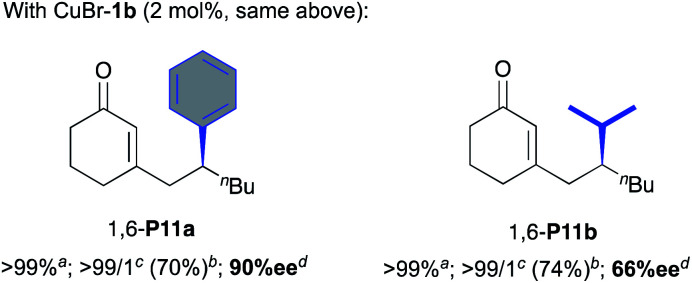
| 4 | CuBr-1b (2) | >99 (68) | >99 : 1 | 85 |
| 5 | CuBr-1a (2) | > 99 (90) | >99 : 1 | 65 |
| 6 | 2b (5) | Nr | Nd | Nd |
| 7 | 2a (5) | Nr | Nd | Nd |
| ||||
Conversions were monitored by TLC.
Isolated yields after silica gel chromatography.
Ratios between 1,6 : 1,4-adducts were determined by 1H NMR spectroscopy.
Enantiomeric excesses were determined by chiral-phase GC or HPLC analysis.
Reaction conditions: (1) L1bH·PF6 (3 mol%), n-BuLi (8 mol%), Cu(OTf)2 (2 mol%), THF, 0 °C, Et2Zn, then S10, rt. (2) DBU (1 equiv.), CH2Cl2, rt, 5 h.
Large amount of side products were formed. Mr = Messy reaction. Nd = not determined. Nr = no reaction.
