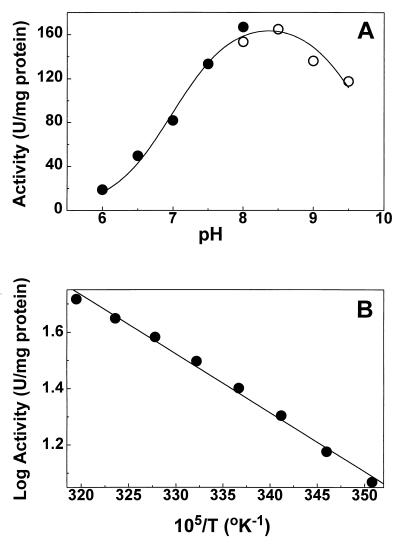
FIG. 6.
Effect of pH and temperature on the activity of BADH from P. aeruginosa. (A) Profile of velocity versus pH. Enzyme (0.5 μg/ml) activities were determined as described in Materials and Methods with 100 mM potassium phosphate (●) or 100 mM potassium pyrophosphate (○) buffer. The points are the experimentally determined values, and the line drawn through these points was calculated from the best fit of the data to equation 3. (B) Arrhenius plot of log of specific activity versus reciprocal of absolute temperature. The same amount of enzyme (2 μg/ml) was assayed under the standard conditions in 100 mM potassium phosphate buffer (pH 8.0) at the different fixed temperatures without preincubation.