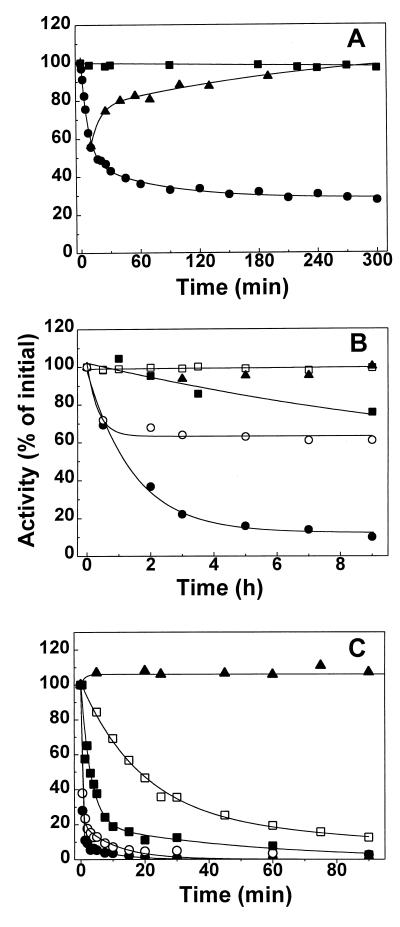
FIG. 7.
Effect of temperature, dilution, and buffer composition on the stability of BADH from P. aeruginosa. (A) Kinetics of the reversible heat inactivation. The enzyme (31 μg/ml) was incubated at 40°C in 10 mM potassium phosphate buffer (pH 8.0) containing 0.1 mM EDTA, 5 mM β-mercaptoethanol, 20% (wt/vol) sucrose, and 25 mM KCl in the absence (●) or presence (■) of 0.5 mM NADP+. After the times indicated, 4-μl samples were withdrawn and immediately added to the standard assay mixture. Reactivation of the heated enzyme (▴) was achieved by cooling the partially inactivated enzyme at 30°C. (B) Kinetics of the inactivation by dilution. Enzyme was diluted to 0.3 (●, ○, and ▴) or 3 (■ and □) μg/ml in 100 mM potassium phosphate buffer at pH 8.0 (●, ○, ■, and □) or pH 6.5 (▴) in the absence (●, ■, and ▴) or presence (○ and □) of 0.38 mM NADP+. The enzyme was incubated at 30°C and assayed at various time points to determine the activity remaining, using the standard assay. (C) Kinetics of inactivation in the absence of K+ ions. Enzyme (31 μg/ml) was incubated at 30°C in 31 mM Tris-HCl buffer (pH 8.0) in the absence of KCl and NADP+ (●) or in the presence of 25 mM KCl (○), 200 mM KCl (■), 0.5 mM NADP+ (□), or 0.5 mM NADP+ plus 25 mM KCl (▴) and was assayed at various time points to determine the activity remaining. In all panels the results are plotted as percentages of that for the untreated control. The lines are the results of the best fits of the experimental data to equation 4 (A) or to equation 5 (B and C).