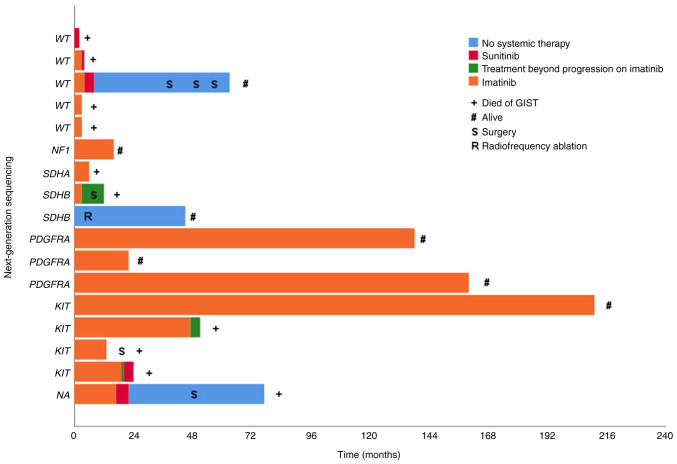
Figure 1.
Clinical outcome in 17 KIT/PDGFRA/BRAF wild-type GIST patients (as assessed by standard methods reverse transcription-quantitative PCR and Sanger sequencing) and their next-generation sequencing molecular status. Patients are listed according to Table III. KIT, KIT proto-oncogene, receptor tyrosine kinase; PDGFRA, platelet-derived growth factor receptor alpha; GIST, gastrointestinal stromal tumors.