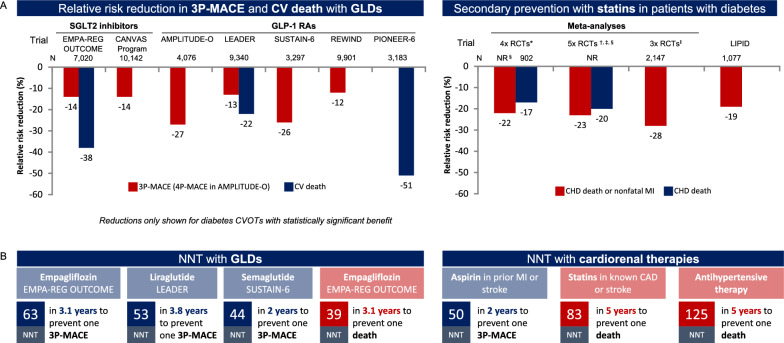
Fig. 4.
Diabetes CVOTs in the broader context of cardiology trials. PIONEER-6 was a small study (N = 3183) of short duration, designed to rule out excess risk of 3P-MACE, and not powered to demonstrate superiority. Certain diabetes CVOTs have shown cardiorenal protective effects that may arguably be comparable to outcomes with cardiorenal therapies [116], such as the relative risk reduction of CV events compared with statins [27, 30–32, 34, 143, 144] (A), or NNT to prevent CV events compared with statins, aspirin or antihypertensive therapy [27, 116, 145, 146] (B). For example, patients with diabetes and CVD in the LIPID trial had a 19% reduced risk of CHD death or nonfatal MI over 6 years with the statin pravastatin compared with placebo [143]; meta-analyses of secondary prevention in patients with diabetes in multiple statin trials have produced similar results [143, 144, 147]. 3P-MACE, 3-point major adverse CV event; CHD, coronary heart disease; CV, cardiovascular; CVOT, CV outcomes trial; GLD, glucose-lowering drug; GLP-1 RA, glucagon-like peptide-1 receptor agonist; MI, myocardial infarction; NNT, number needed to treat; NR, not reported; RCT, randomised control trial; SGLT2, sodium–glucose transporter 2. *Four RCTs (4S, CARE, Post-CABG and VA-HIT) for CHD death and nonfatal MI, and 3 RCTs (4S, CARE, Post-CABG) for CHD death. †Five RCTs (4S, CARE, LIPID, Post-CABG and VA-HIT). ‡Includes 1 RCT that investigated a non-statin cholesterol-lowering drug. ‖Three RCTs (4S, CARE, LIPID)