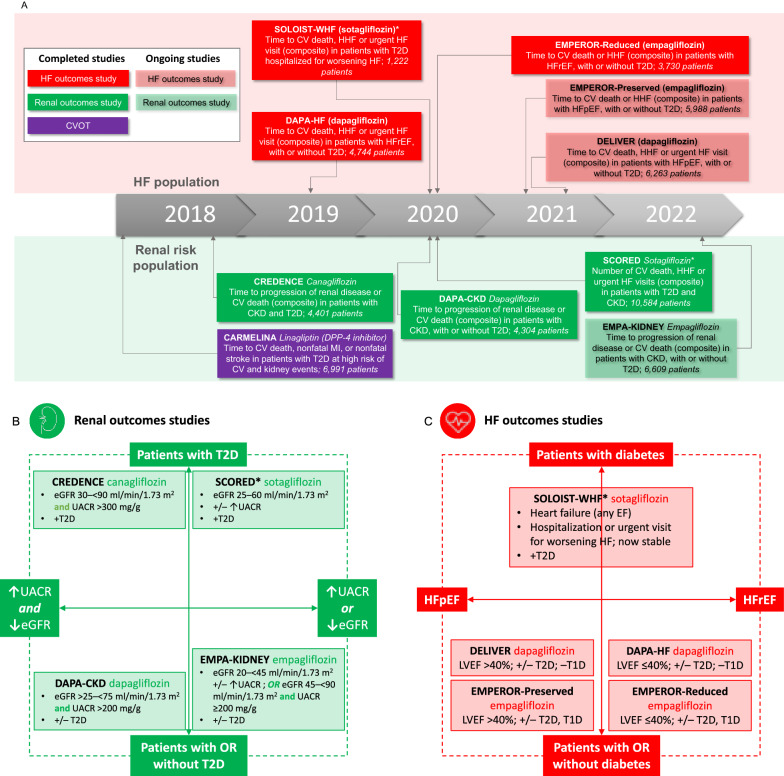
Fig. 5.
Completed and ongoing studies of SGLT2 inhibitors (and linagliptin) in renal risk or HF populations. Secondary HF and renal outcome measures in diabetes CVOTs of SGLT2 inhibitors were hypothesis generating, suggesting possible protective events on HF and renal disease. Only one diabetes CVOT (CARMELINA) included a majority of patients with CKD [101] (A); however, this was a study not on an SGLT2 but on a DPP-4 inhibitor, linagliptin, and was designed to demonstrate CV safety in a renal risk population, and not renal protection [101]. Subsequently, several dedicated HF [18, 69, 71, 148, 149] and renal [36, 68, 72, 150] outcome studies have been completed, or are underway, including studies that include patients with HF (B) or CKD (C) without diabetes [63, 68, 69, 71, 86, 148, 149, 151]. Among HF studies, both HFrEF [18, 69, 71] and HFpEF [18, 86, 148, 149] have recently or are being investigated (B), while renal studies include populations with albuminuria and/or with impaired renal function [36, 68, 72, 150] (C). −, without; +, with; +/−, with or without; CKD, chronic kidney disease; CV, cardiovascular; eGFR, estimated glomerular filtration rate; HF, heart failure; HFp/rEF, HF with preserved/reduced ejection fraction; HHF, hospitalisation for HF; LVEF, left ventricular ejection fraction; T1/2D, type 1/2 diabetes; UACR, urinary albumin–creatinine ratio. Source for study completion dates, prespecified endpoints, enrolment numbers and inclusion criteria: clinicaltrials.gov. *SOLOIST-WHF was terminated early