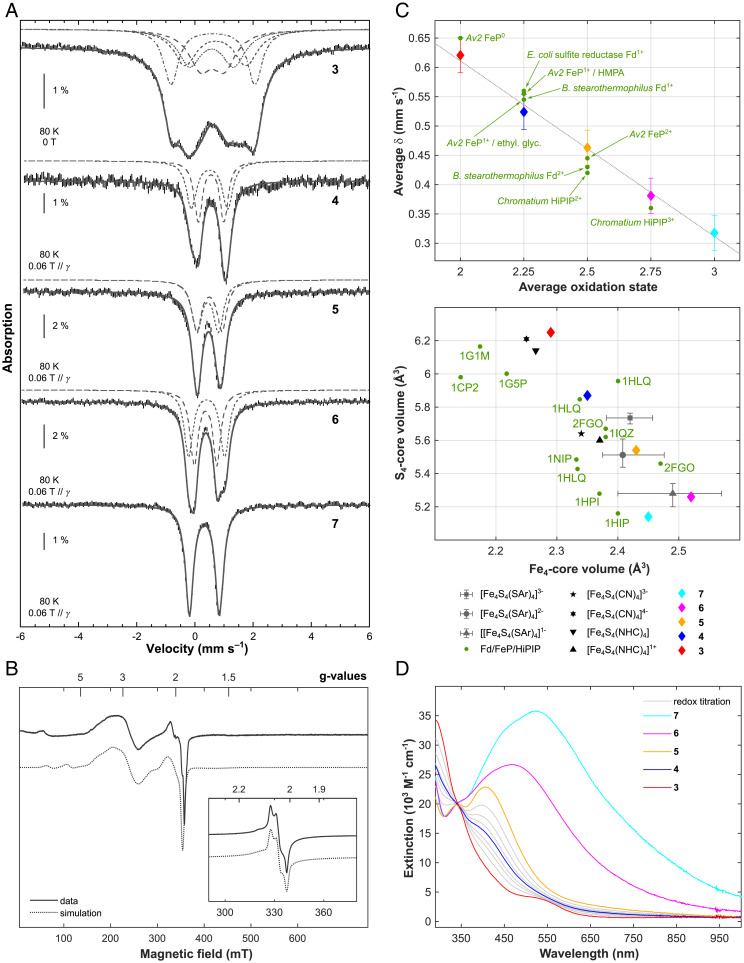
Fig. 4.
57Fe Mössbauer, EPR, UV-vis electronic absorption and geometric data for clusters 3–7. (A) Mössbauer spectra (hatched bars) recorded on powder samples at 80 K with a 0.06-T external magnetic field applied parallel to the γ-beam for 4–7 and at zero-field for 3. Simulations are overlaid as gray solid lines and deconvolutions are displayed above. See SI Appendix, Table S2, for the parameter values. Note that for the Mössbauer spectrum of 7, an impurity accounting for 4% of the total Fe content has been subtracted (δ = 0.96 mm s−1, ΔEQ = 2.09 mm s−1, Γfwhm = 0.25 mm s−1). (B) X-band perpendicular mode EPR spectra of 2 mM toluene solutions of 4, recorded at 10 K and 6 (Inset), recorded at 40 K. Data are represented by solid lines and simulations by dotted ones. For details of the fitting parameters, refer to SI Appendix, Figs. S24 and S25. (C, Top) Averaged value of the isomer shift issued from the simulations of the 80-K Mössbauer spectra upon the averaged oxidation state of the iron ions in complexes 3–7. The gray dotted line is a linear fit of the four experimental points. Data recorded on selected biological systems are shown as green dots. (C, Bottom) Variation in the Fe4 and S4 core volumes of selected FeS cubane containing structures of biological (FeP, 4Fe-4S Fds, and HiPIP) and synthetic origin. Synthetic models of aromatic thiolate supported cubanes span 8, 11, and 5 examples for [Fe4S4(SAr)4]1–, [Fe4S4(SAr)4]2–, and [Fe4S4(SAr)4]3–, respectively. The data are represented by a gray triangle, dot, and square in the position of the arithmetic mean. Bars indicate the maximum and minimum values reported. Data for the redox series 3–7 are shown by red, blue, yellow, magenta, and cyan diamonds with error bars; refer to SI Appendix, Table S12, for all values and references. (D) UV-vis electronic absorption spectra of 1.1 · 10−4 M toluene solutions of compounds 3 (red), 4 (blue), 5 (yellow), 6 (magenta), and 7 (cyan). Solid gray lines indicate spectra measured along the stoichiometric redox comproportionation reaction between 3 and 5, which results in the formation of 4.