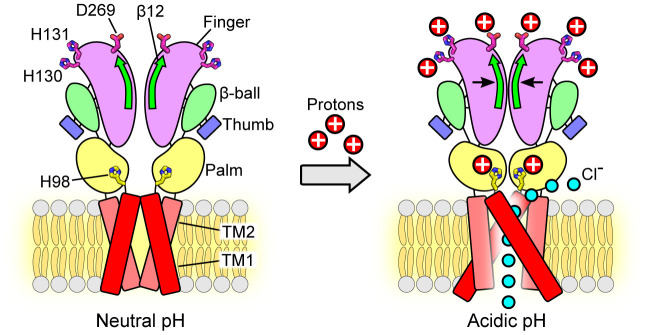
Fig. 7.
Schematic representation of the conformational changes during pH-dependent activation and allosteric gating of the PAC channel. Representative cartoon of PAC, depicting a dimer of the homotrimeric subunit, in the closed and open states. Each subunit is composed of two TMDs, N and C termini, and a large ECD. In the closed state, TM1 and TM2 run parallel and interact with each other. At pH 4, TM1 swings and interacts with TM2 of the adjacent subunit. The ECD contracts toward the pore axis, resulting in a shorter overall structure and a more compact ECD in comparison to that at pH 8. The joint region is at the intersubunit interface and connects the upper finger domain to the lower palm domain. The potential proton-binding sites (H130, H131, D269) are located at the top of the ECD, distal to the ion-conducting pore and channel gate. Protonation of these sites allosterically affects the state of channel gate leading to the PAC channel activation. The β12 strand and the joint region allosterically transduce the pH-dependent conformational changes from the peripheral proton-binding sites to the channel pore. Therefore, combining the available structural information, we propose a model in which protonation of residues in the extracellular finger domain induces the contraction of the ECD, which, together with the conformational change of H98 in the ECD–TMD interface, leads to the opening of the ion-conducting pore. The permeation of Cl− ions through the fenestration site is illustrated (15).