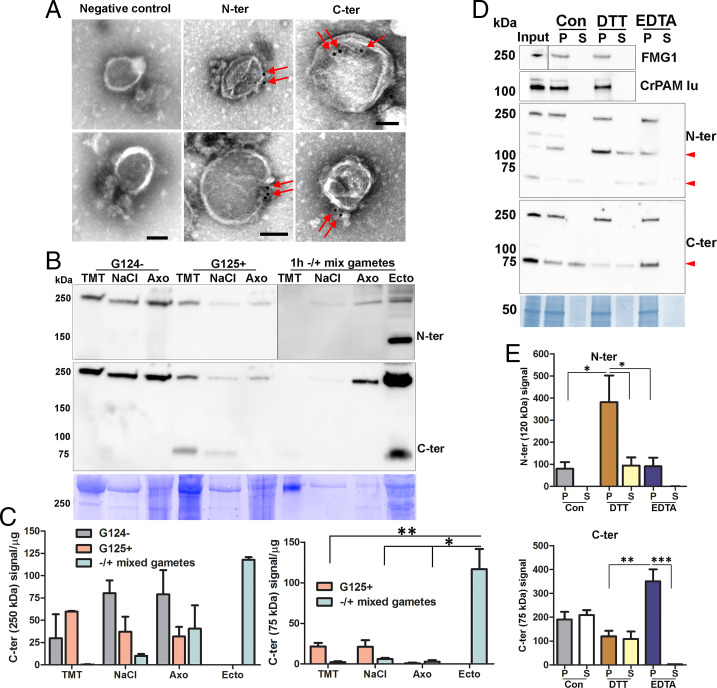
Fig. 7.
Ciliary localization and association of proGATI and its fragments with ectosomes. (A) Whole-mount negative-stain immunogold EM images of intact ectosomes incubated with affinity-purified N-ter or C-ter antibodies and a gold-tagged secondary antibody; both epitopes localized to the ectosomal surface. Ectosomes incubated with gold-tagged secondary anti-rabbit antibody alone served as a negative control. (Scale bars, 100 nm.) (B) Cilia were sequentially treated with buffers containing 1% Triton X-100 (TMT) and 0.6 M NaCl (NaCl); the resulting axonemal pellet (Axo) was solubilized in 1% SDS-buffer. The subciliary fractions from resting minus (G124−) and plus (G125+) gametes, mixed gametes, and mating ectosomes (Ecto) were fractionated by SDS/PAGE, blotted and probed with affinity-purified N-ter and C-ter antibodies. Equal amounts of protein (20 µg) were loaded for each sample. (C) Immunoblot quantification of the 250- and 75-kDa C-ter products. Means are average of duplicates and error bars indicate ± range, where *P < 0.05, **P < 0.01. (D) Freshly isolated mating ectosomes (Input) were washed with buffer alone (10 mM Hepes, control) or with buffer containing 10 mM DTT or 10 mM EDTA; after centrifugation, the resulting supernatants (S) and pellets (P) were analyzed for the presence of proGATI (using N-ter and C-ter antibodies), PAM, and FMG1. Red arrowheads mark the 120-, 75-, and 63-kDa bands. Samples loaded represent the pellets and corresponding supernatants derived from an initial 15 µg of ectosomes. (E) Quantification of 120-kDa N-ter signal (n = 4) and 75-kDa C-ter signal (n = 3); means ± SEM are shown. Asterisks indicate significant differences between the groups: *P < 0.05, **P < 0.01, ***P < 0.001.