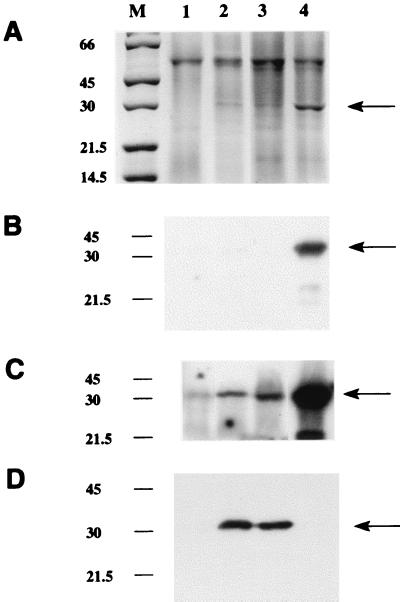
FIG. 3.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis analysis of recombinant NAT proteins in M. smegmatis. Gels were loaded with 15 μl of supernatants from lysates of M. smegmatis transformed with pACE-1 alone (lane 1), with M. tuberculosis nat (lanes 2 and 3), or with M. smegmatis nat (lane 4) (M, molecular mass markers [in kilodaltons]). (A) Staining with Coomassie blue. The arrow indicates the additional band in the supernatants transformed with a nat gene. (B to D) Western blots developed by using an enhanced chemiluminescence detection system as described previously (27), where exposure time for detection of the second antibody is either 1 min (B and D) or 20 min (C). In each panel, lane 1 has been loaded with 10 μg of total protein and lanes 2 to 4 have been loaded with 600 ng of total protein. An antiserum raised against recombinant M. tuberculosis NAT protein synthesized in E. coli is shown in panel D. The antiserum used in panels B and C is the antiserum against pure S. typhimurium NAT (24). Both antisera were used at a dilution of 1:100,000.