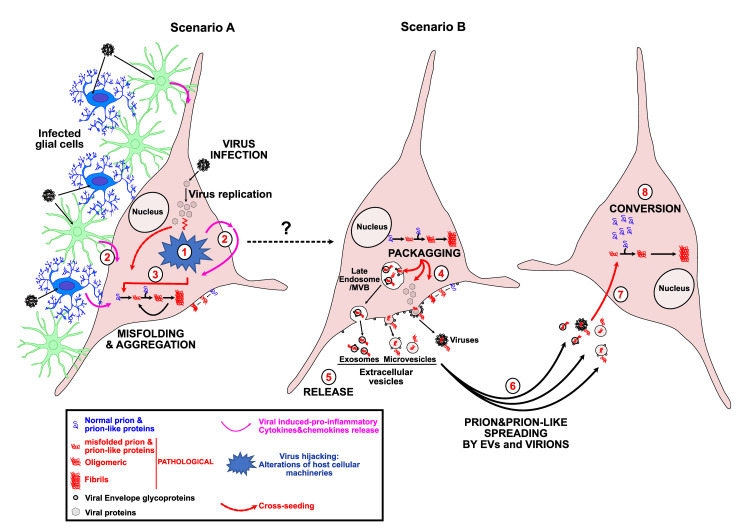
Fig 1. Virus infection and step-by-step misfolding and aggregation, spreading, and prion-like conversion.
Scenario A: Viruses hijack the host cellular machineries and pathways for their own benefit to efficiently drive their replication. To this end, viruses developed different strategies to impair intracellular trafficking like nucleocytoplasmic export, the endo-lysosomal secretory and degradation pathways as well as all the machineries involved in protein quality control and in proteostasis (1). In response to infection, infected host cells, including glial cells, reply through the expression of restriction factors and secretion of proinflammatory cytokines and chemokines (2). Inactivation of protein quality control and induction of neuroinflammation are major events leading to the misfolding and aggregation processes of ND-associated proteins like PrP, α-Synuclein, APP/Aβ, Tau, TDP-43, and FUS (3). Scenario B: Misfolded pathological proteins can have different etiology. They can be induced by aging, by mutations in susceptibility genes or ND-associated genes themselves, or by environmental stressors such as pathogens-like viruses (see dotted black line) or repeated contacts with chemicals. In virus-infected cells, misfolded proteins can be secreted in EVs that bud from the plasma membrane (microvesicles and viral particles) or from the surrounding membrane of MVBs (exosomes) (4). Like viral particles, EVs are released (5) in presence or absence of viral Envelope glycoprotein at their surface, conferring broadened cell tropism and increased endosomal escape essential for efficient spreading (5, 6). Viral particles and EVs containing pathological protein aggregates enter target cells through different mechanisms, including the one mediated by the interaction between the viral Envelope glycoprotein and its host membrane receptor (7). Once introduced into the target cells, protein aggregates induce the conversion of their normal counterpart into aggregated isoforms through a conformational templating mechanism (8). EV, extracellular vesicle; MVB, multivesicular body; ND, neurodegenerative disease.