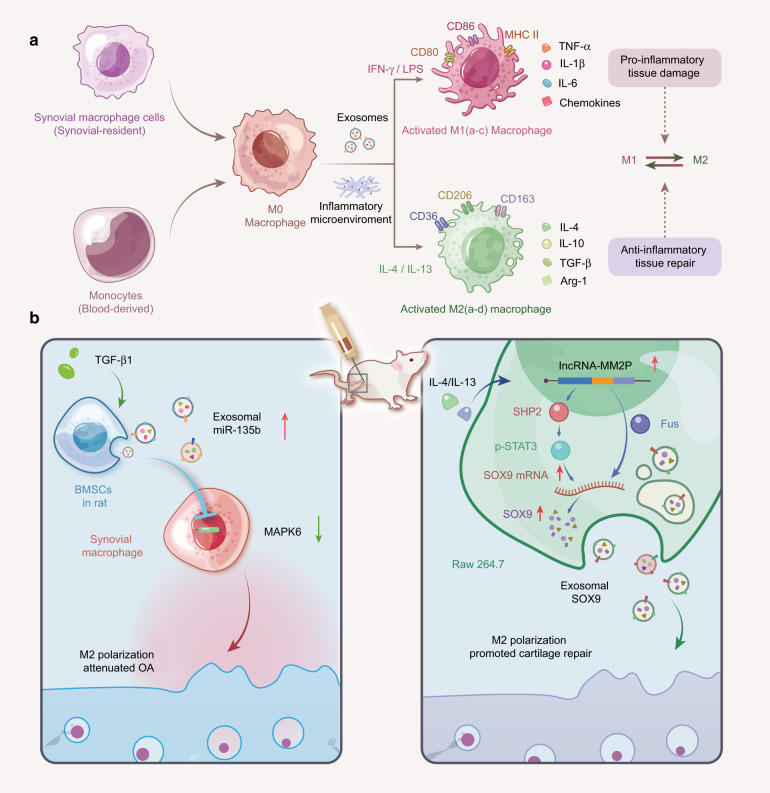
Fig. 5.
Macrophage polarization in cartilage tissue. a The phenotype of macrophage polarization. Synovial macrophages can be derived from monocytes that originally reside in synovial tissue or in the circulation. Evidence suggests that primary macrophages (M0) can be activated and differentiate into the M1 phenotype (INF-γ and LPS stimulation) and M2 phenotype (IL-4 and IL-13 stimulation). Both M1-polarized (subdivided into M1a-c, which promotes the inflammatory response) and M2-polarized (subdivided into M2a-d, which suppresses the inflammatory response) macrophages can regulate the function of cartilage tissue through the secretion of exosomes carrying specific cytokines and chemicals. The conversion of the M2 phenotype into the M1 phenotype can effectively delay OA degeneration. b The role of exosomes in regulating macrophage polarization in OA. Left: TGF-β1-stimulated BMSC-derived exosomes attenuate cartilage injury by promoting M2 polarization of synovial macrophages through the miR-135b/MAPK6 signaling pathway. Right: Upon IL-4 or IL-13 stimulation, lncRNA MM2P is upregulated in rat macrophage (RAW264.7) cells and blocks SHP2-mediated dephosphorylation of STAT3 at Try705, thus promoting the transcription of the SOX9 gene. Alternatively, MM2P interacts with the RNA-binding protein FUS to stabilize SOX9 mRNA. Consequently, the elevated SOX9 mRNA and proteins can be delivered to chondrocytes via exosomes to promote cartilage repair. INF-γ interferon-gamma, LPS lipopolysaccharide, IL-4 interleukin (IL)-4, IL-13 interleukin (IL)-13, TGF-β1 transforming growth factor-beta1, BMSCs bone marrow mesenchymal stem cells, MAPK6 mitogen-activated protein kinase 6, SOX9 SRY-box transcription factor 9, STAT3 signal transducer and activator of transcription 3, ncRNAs noncoding RNAs, OA osteoarthritis