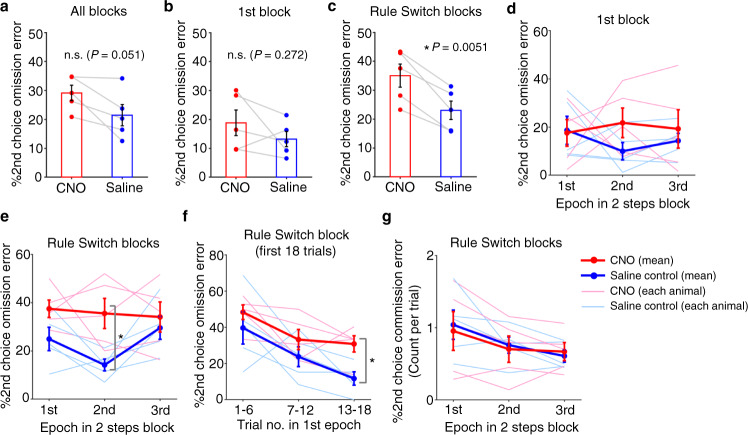
Fig. 4. Chemogenetic silencing of ACC neuronal terminals in M2 disrupted animals’ choice performance.
a Group result of 2nd choice performance in 2 steps condition with a local infusion of either saline or CNO solution. b Same as in a, but %2nd choice omission error for trials in 1st block (i.e., non-rule switching block). c Same as in a and b, but %2nd choice omission error for trials in Rule Switch-blocks. d 2nd choice performance was plotted separately for three epochs in the 1st block for sessions with a local infusion of saline or CNO solution. Thick red and blue lines represent across-animal averages of CNO and saline conditions, respectively (n = 5 rats). Thin lines represent individual animals. 1st, 2nd, and 3rd epochs correspond to 1–18th, 19–36th, and 37–55th trials. Neither CNO dose nor epoch in 2 steps rule block showed a main effect (P > 0.2 for CNO dose and P > 0.9 for epoch). e Same format as in d, but for Rule Switch-blocks. CNO dose showed a significant main effect (P = 0.00402, F1,26 = 9,969) while epoch did not (P > 0.3). Post hoc comparisons were conducted using paired t-test (two-sided) with Bonferroni’s correction across epochs (such correction was not conducted for dose because there are only two conditions, i.e., saline and CNO conditions). *P = 0.0483. f 2nd choice performance in 2 steps condition (%2nd choice omission error) was plotted separately for 1–6th, 7–12th, and 13–18th trials in the 1st epoch of Rule Switch-blocks. *P = 0.0432. g Average number of 2nd choice commission error per trial was plotted separately for three epochs in Rule Switch-blocks of 1 step condition. Repeated measures two-way ANOVA with both CNO dose and epoch being within-subject factors revealed no main effect of CNO (P > 0.8). Epoch showed a moderate effect but did not reach statistical significance (P = 0.105, F2,26 = 2.461, n = 5 rats). All pairwise comparisons were conducted using a two-sided paired t-test with n = 5 rats (a–c, e, f). Error bars, SEM (a–g). Source data are provided as a Source Data file.