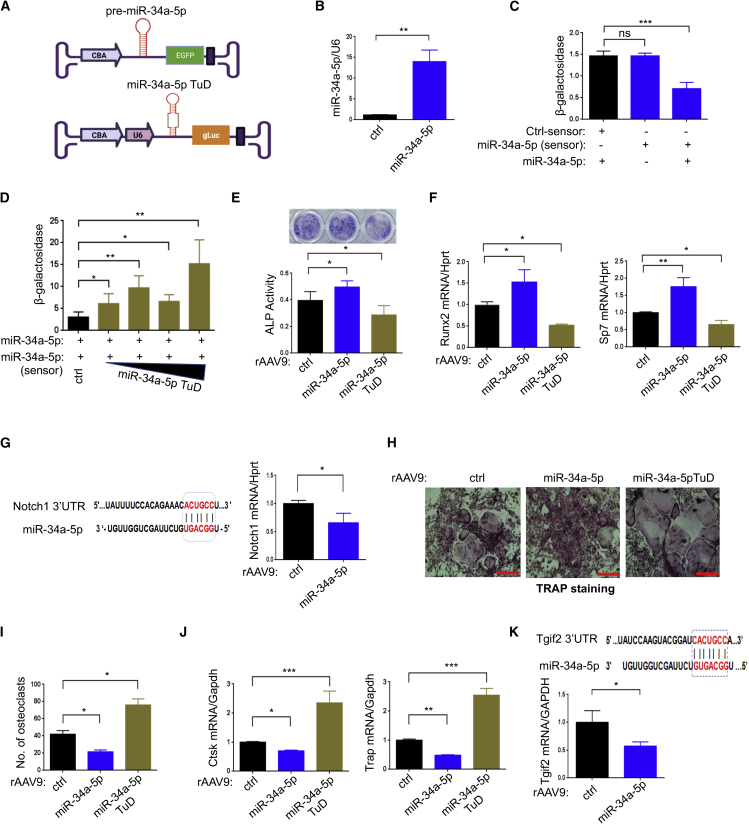
Figure 2.
Effects of rAAV9 carrying miR-34a-5p or miR-34a-5p TuD on osteoblast or osteoclast differentiation
(A) Diagram showing AAV vector genome that contains miR-34a-5p and EGFP gene (top) or miR-34a-5p TuD and gLuc reporter gene (bottom). (B) A control (ctrl) or miR-34a-5p plasmid was transfected into HEK293 cells and, 48 h later, expression of miR-34a-5p was measured by RT-PCR and normalized to U6. (C) HEK293 cells were transfected with ctrl-sensor or miR-34a-5p-sensor plasmid in the absence or presence of miR-34a-5p plasmid and, 48 h later, β-galactosidase activity was measured and normalized to firefly luciferase. (D) The miR-34a-5p plasmid was transfected into HEK293 cells along with the miR-34a-5p-sensor plasmid with increasing concentrations of miR-34a-5p TuD plasmid. After 48 h, β-galactosidase activity was measured and normalized to firefly luciferase. (E–G) Mouse BMSCs were transduced with rAAV9 carrying ctrl, miR-34a-5p, or miR-34a-5p TuD for 2 days and cultured under osteogenic conditions. ALP staining and activity (E) and expression of Runx2 and Sp7 (F) were assessed at day 6 of culture. (G) Computational analysis showing the complementarities of miR-34a-5p to the 3′ UTR of Notch1 (left). mRNA levels of Notch1 in miR-34a-5p-expressing BMSCs were assessed by RT-PCR (right). (H–K) Two days after treatment with M-CSF and RANKL, BMMs were transduced with rAAV9 carrying ctrl, miR-34a-5p, or miR-34a-5p TuD, cultured under osteoclast differentiation conditions, and stained for TRAP. Representative images of TRAP-stained osteoclasts (H) and numbers of TRAP-positive osteoclasts were quantitated (I). mRNA levels of Ctsk and Trap were measured by RT-PCR and normalized to Gapdh (J). (K) The predicted consequential pairing of Tgif2 3′ UTR with miR-34a-5p is shown (top) and Tgif2 expression as measured by RT-PCR (bottom). Scale bar, 400 μm in (H). Values represent mean ± SD: ns, not significant; ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001, ∗∗∗∗p < 0.0001 by an unpaired two-tailed Student’s t test (B, G, and K) and one-way ANOVA test (C–F, I, and J).